
Concept explainers
Interpretation:
The name and structure of the product obtained from the ionic addition of IBr to propene is to be given.
Concept Introduction:
Substitution reaction: A reaction in which one of the hydrogens of a hydrocarbon or a functional group is substituted by any other functional group.
Elimination reaction: A reaction in which two substituent groups are detached and a double bond is formed.
Nucleophilic substitution reaction: A reaction in which one of the hydrogens of a hydrocarbon or a functional group is substituted by any nucleophile.
Addition reaction: A reaction in which two or more than two unsaturated compounds react to form a product.
Answer to Problem 1PP
Solution:
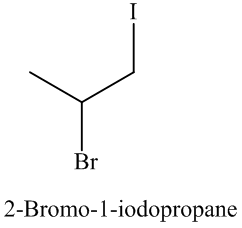
Explanation of Solution
The unsaturated compound propene undergoes addition reaction on reacting with
The reaction is as follows:
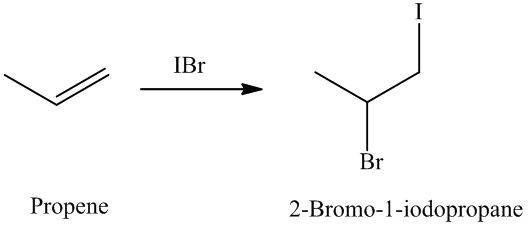
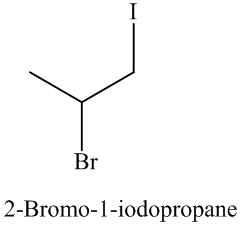
The name and structure of the product formed is as:
Want to see more full solutions like this?
Chapter 8 Solutions
Organic Chemistry
- Starting with an alcohol in each case outline the synthesis of isobutylene by two different routes.arrow_forwardWrite the overall balanced equation and mechanism for the transformation of bromobenzene into benzoic acid through a Grignard reaction. Include all reagents and products but not solvents.arrow_forwardSuggest reactivity of compound A, B and C in increasing order of E2 reactionarrow_forward
- Suggest and explain two synthetic methods for the conversion of compound 6 to compound 4. Include side reactionsarrow_forwardAlthough derivatization of a reaction should be avoided as much as possible according to the principles of green chemistry, the protective groups needed in organic synthesis mainly regulate the chemoselectivity of the reaction. Mention only 3 requirements for a good protective group !arrow_forwardProvide a reasonable synthetic strategy for the synthesis of a racemic mixture of (1R,2R) and (1S,2S)-2-bromo-1-methylcyclopentanol from the compound shown below... Provide the bond line structure for the major organic products obtained in each step of the proposed strategyarrow_forward
- Starting with acid chloride with exactly 5 carbon atoms, and using appropriate reagents outline the synthesis of the following molecules:arrow_forwardWrite the mechanism for the ionic addition of HBr to 2-methylpropene and write the mechanism for the ionic addition of HBr to 2-methylpropene in the presence of dialkyperoxides and compare both, state any specific diffarrow_forward#12. Outline a synthesis of the following molecules starting with ethyne and CH2I2 as the only carbon sources and any needed inorganic reagents. Obviously more than one ethyne molecule is needed for each of the syntheses.arrow_forward
- Provide a mechanism which explains the following conversions. Include all intermediate structures where appropriate and watch your arrows and chargesarrow_forwardShow by writing a suitable series of steps of equations how you would prepare 1-Hexene from 1-butene and Acetylene. Include all necessary organic and inorganic reagents.arrow_forwardUsing 1-bromobutane and any organometallic reagent among those seen in class, suggest the synthesis for each of the following compounds: A. 1-phenyl-1-hexanol b. 1-butylciclobutanolarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





