
Concept explainers
(a)
Interpretation:
The standard enthalpy of reaction has to be calculated using the values of bond dissociation enthalpies.
Concept Introduction:
The value of standard enthalpy change
Where,
(a)
Explanation of Solution
Given reaction (1):
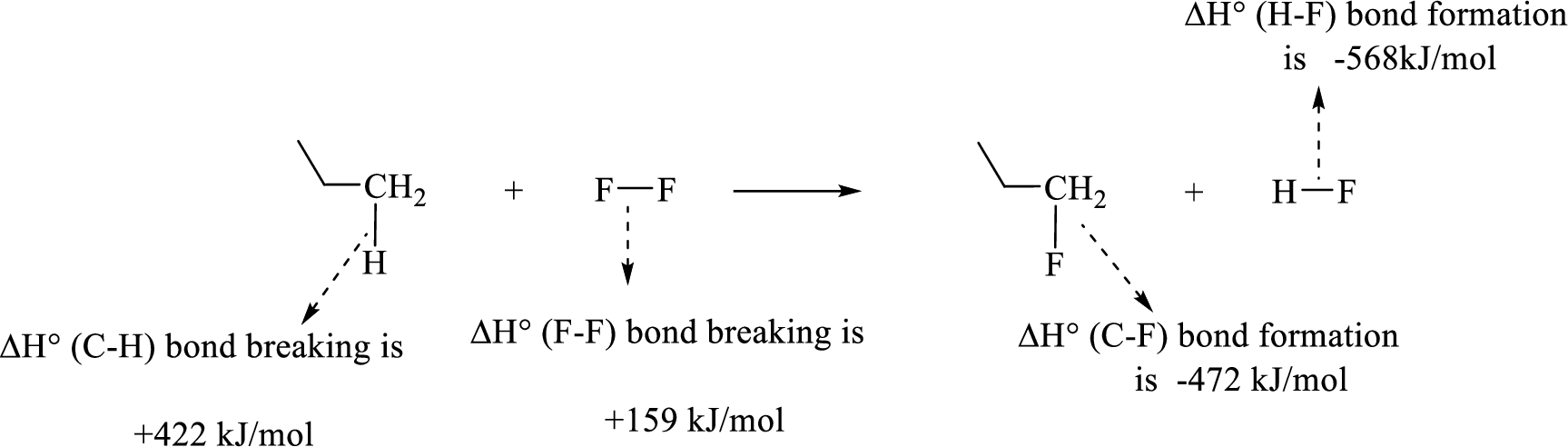
The reaction is given below:
Therefore, standard enthalpy of given reaction is
Given reaction (2):
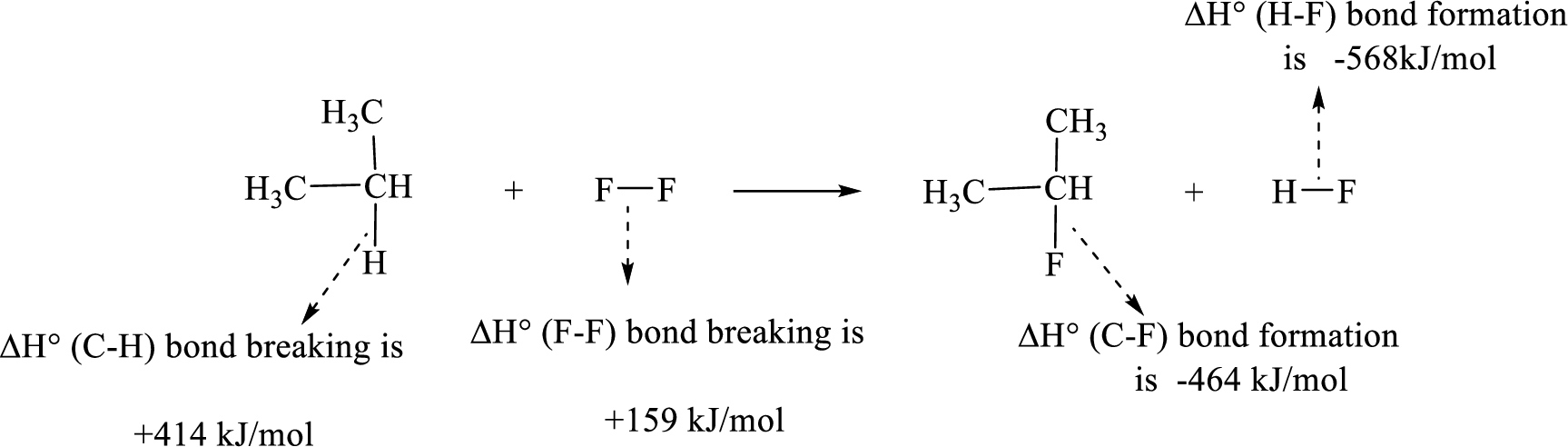
The reaction is given below:
Therefore, standard enthalpy of given reaction is
(b)
Interpretation:
A pair of chain propagation steps for each reaction has to be proposed; the value of
Concept Introduction:
Radical Bromination: A type of halogenation reaction in which the bromine atom gets bonded with the
Radical reaction of
Stability of Radicals: Radicals are highly unstable due to its unpaired valence electron of an atom.
The increasing order of radical stability is given below,
Benzylic > allylic > tertiary > secondary > primary > methyl
Radical chain reaction:
Initiation reaction: 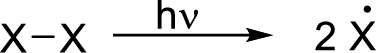
Chain propagation: 
Chain termination:
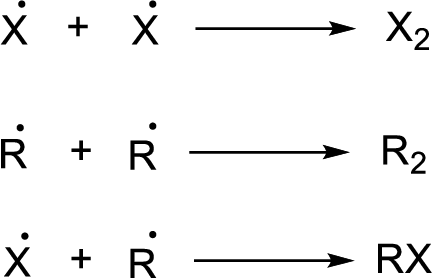
(b)
Explanation of Solution
Given reaction (1):
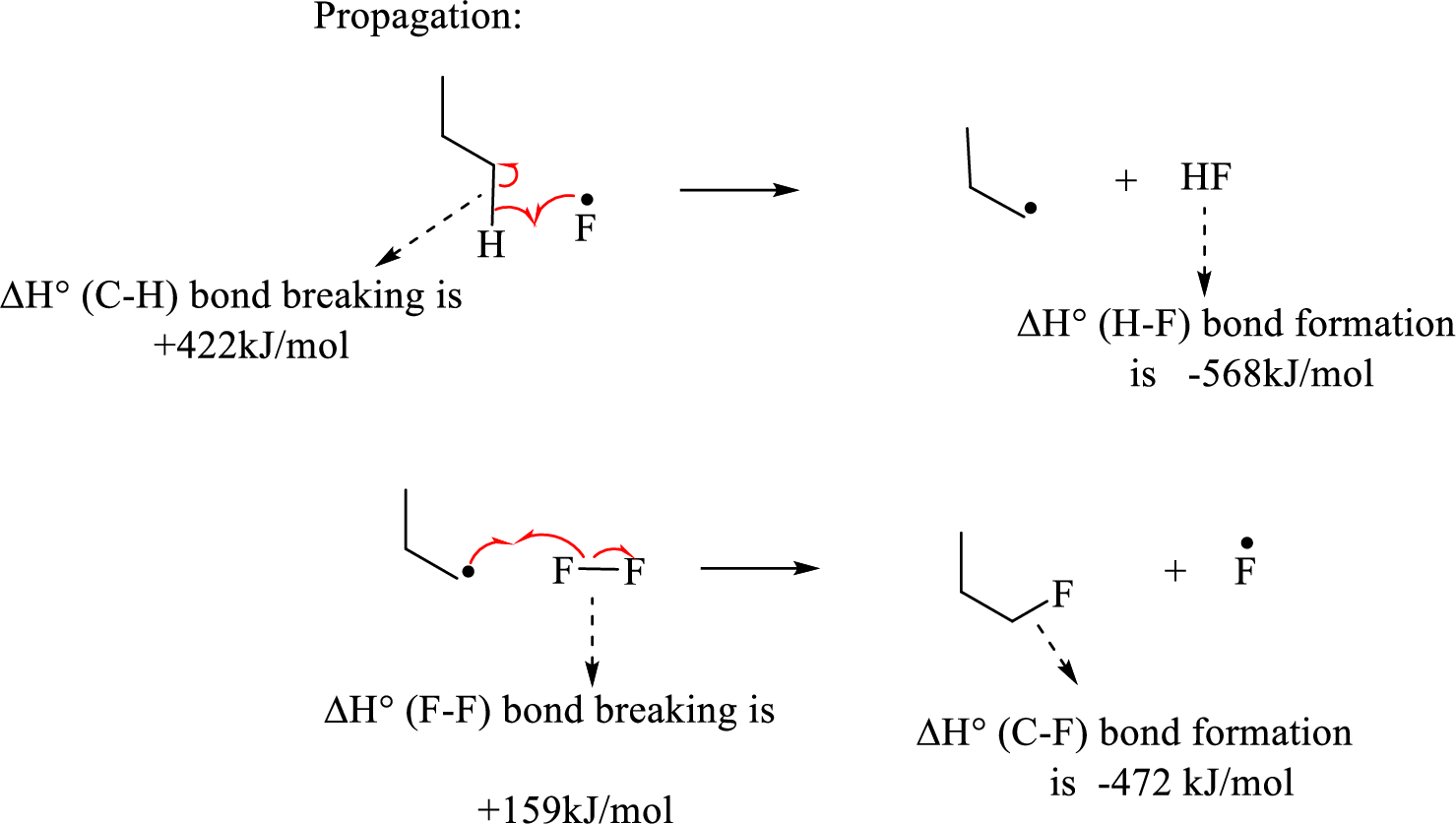
The propagation step involves extremely reactive fluorine radicals react towards the propane by abstracting the terminal hydrogen forming primary radical and
Hence, the pair of propagation steps is shown above.
Given reaction (2):
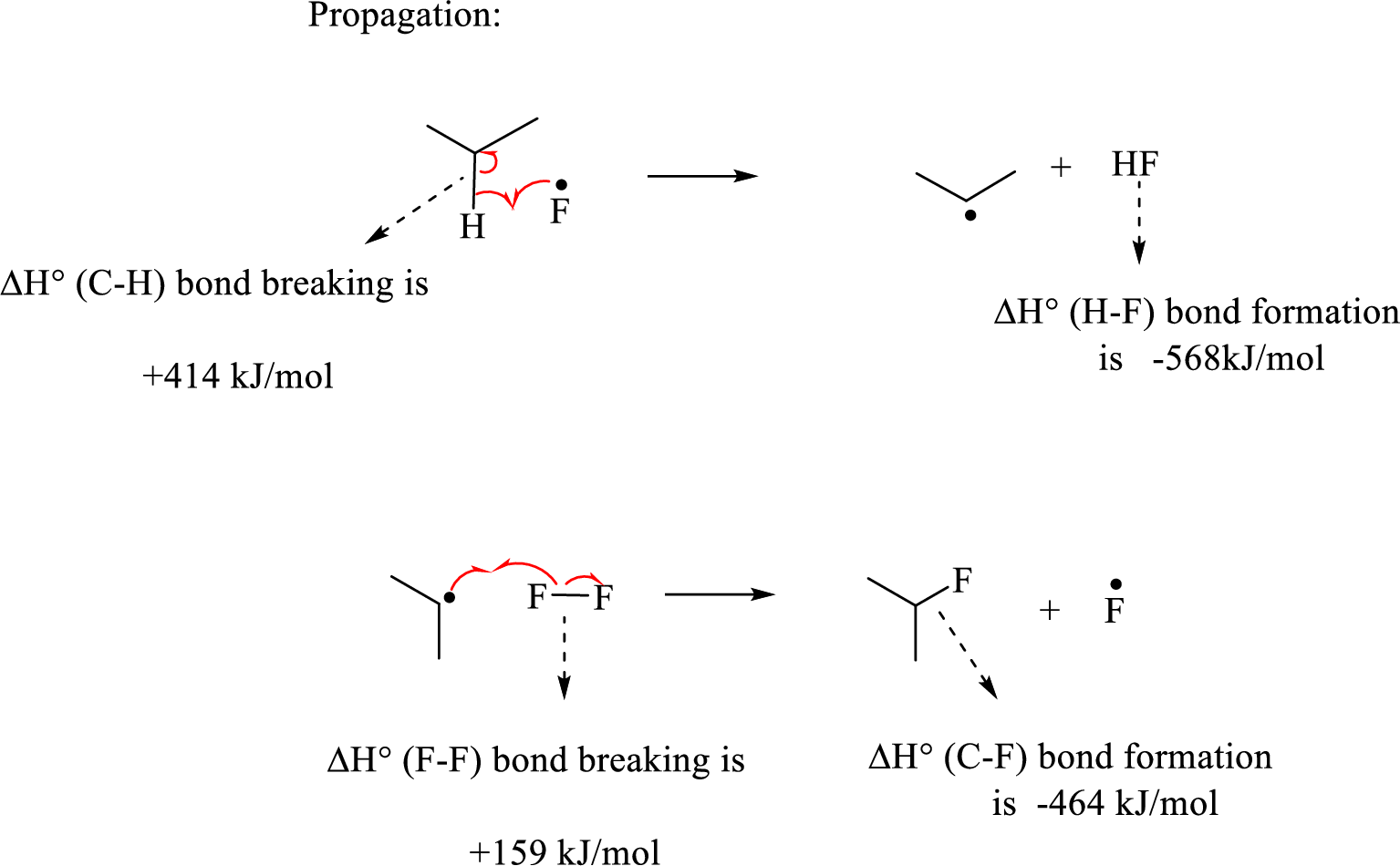
Initiation step involves the homolytic bond cleavage of fluorine molecule by irradiation of light or heat leads to the formation of two Fluorine radicals.
The propagation step involves extremely reactive fluorine radicals react towards the propane by abstracting the secondary hydrogen forming secondary radical and
Hence, the pair of propagation steps is shown above.
Therefore, for each reaction step the value of
(c)
Interpretation:
The regioselectivity of radical fluorination relative to that of radical chlorination and Bromination has to be predicted using Hammond’s postulate.
Concept Introduction:
Radical Bromination: A type of halogenation reaction in which the fluorine atom gets bonded with the alkane or alkyl substituents resulting to the product with added fluorine atom via radical mechanism.
Radical reaction of
Stability of Radicals: Radicals are highly unstable due to its unpaired valence electron of an atom.
The increasing order of radical stability is given below,
Benzylic > allylic > tertiary > secondary > primary > methyl
Radical chain reaction:
Initiation reaction: 
Chain propagation: 
Chain termination:
(c)
Explanation of Solution
In each radical fluorination sequence, the hydrogen abstraction step is highly exothermic, thus the transition state is reached too early and the transition state more closely resembles the reactant; so the intermediate has very little radical character of product. Therefore, the relative stabilities of primary versus secondary radical is of little of importance in determination of product.
Want to see more full solutions like this?
Chapter 8 Solutions
Organic Chemistry
- Write a conformational structure for 1,2,3-trimethylcyclohexane in which all the methyl groups are axial and then show its more stable conformation.arrow_forwardUsing the table of bond dissociation enthalpies , calculate ▲H° for bromination of propane to give 2-bromopropane and hydrogen bromide.arrow_forwardWhen exactly 1 mole of methane is mixed with exactly 1 mole of chlorine and light is shone on the mixture, a chlorination reaction occurs. The products are found to contain substantial amounts of di-, tri-, and tetrachloromethane, as well as unreacted methane. Explain how a mixture is formed from this stoichiometric mixture of reactants, and propose mechanisms for the formation of these compounds from chloromethane.arrow_forward
- Draw the structure of the organic product of the reaction between cyclohexene and H2SO4, H2O. Use the wedge/hash bond tools to indicate stereochemistry where it exists. Separate multiple products using the + sign In cases where there is more than one answer, just draw one. Please make your answer clear. I did not understand the first answer because it was hand -writtenarrow_forward1. Write bond-line formulas for (a.) four aldehydes with the formula of C5H10O (b) three ketones that have the formula C5H10O (c.) four carboxylic acids with the formula C5H10O2 (d.) three esters with the formula C5H10O2arrow_forwardDraw the structure of the organic product of the reaction between cyclohexene and H2SO4, H2O. Use the wedge/hash bond tools to indicate stereochemistry where it exists. Separate multiple products using the + sign In cases where there is more than one answer, just draw one.arrow_forward
- Acetonitrile (CH3CN) has resonance at 1.97 ppm, whereas methyl chloride (CH3Cl) has resonance at 3.05 ppm, even though the dipole moment of acetonitrile is 3.92 D and that of methyl chloride is only 1.85 D. The larger dipole moment for the cyano group suggests that the electronegativity of this group is greater than that of the chlorine atom. Explain why the methyl hydrogens on acetonitrile are actually more shielded than those in methyl chloride, in contrast with the results expected on the basis of electronegativityarrow_forwardCarbon–carbon bond dissociation enthalpies have been measured for many alkanes. Identify the alkane in each of the following pairs that has the lower carbon–carbon bond-dissociation enthalpy, and explain the reason for your choice. (a) Ethane or propane (b) Propane or 2-methylpropane (c) 2-Methylpropane or 2,2-dimethylpropane (d) Cyclobutane or cyclopentanearrow_forwardAn unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent of H2 over a palladium catalyst. Hydrocarbon A also reacts with OsO4 to give diol B. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH2CO2H, and the other fragment is ketone C. What are the structures of A, B, and C? Write all reactions, and show your reasoning.arrow_forward
- 2-chloropropane is a major product of the reaction of chlorine with propane under ultraviolet light. Write the mechanism for this reaction including the initiation step and the two propagation steps.arrow_forwardYou are required to synthesize 2-bromopentane from the reaction between an alkene with HBr. Which alkene, 1-pentene or 2-pentene, should you react with HBr in order to get 2-bromopentane? Give an explanation.arrow_forwardfluorination of alkanes is highly exothermic. Per Hammond’s postulate, assume that the transition state for radical fluorination is almost identical to the starting material. Assuming this fact, estimate the fraction of each monofluoro product formed in the fluorination of 2-methylbutane.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

