
Concept explainers
(a)
Interpretation:
A second chain propagation step in the given reaction has to be proposed.
Concept introduction:
Halogenation of
Radical chain reaction:
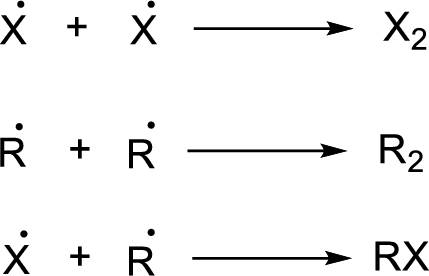
Initiation reaction: 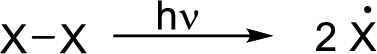
Chain propagation: 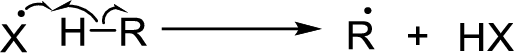
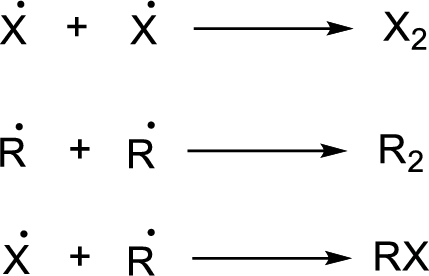
Chain termination:
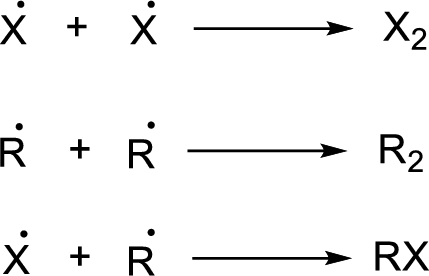
(b)
Interpretation:
Heat of the reaction,
Concept introduction:
Halogenation of alkanes: The replacement of one or more hydrogen atoms by halogen. When alkanes is heated or irradiated with the light of specific wavelength, the alkyl halide are formed by radical chain reaction.
Radical chain reaction:
Initiation reaction: 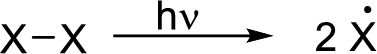
Chain propagation: 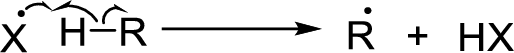
Chain termination:
It is a change in enthalpy of a homolysis reaction at absolute zero where a molecule is broken down into two free radicals.
(c)
Interpretation:
The energies and relative rates of the set chain propagation steps in section 8.5B with given reaction has to be compared.
Concept introduction:
Halogenation of alkanes: The replacement of one or more hydrogen atoms by halogen. When alkanes is heated or irradiated with the light of specific wavelength, the alkyl halide are formed by radical chain reaction.
Radical chain reaction:
Initiation reaction: 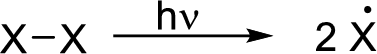
Chain propagation: 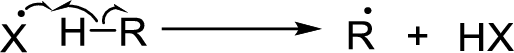
Chain termination:
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry
- a) How many monochlorination products can be obtained from the radical chlorination of methylcyclohexane? Disregard stereoisomers. b) Which product would be obtained in greatest yield? Briefly explain. c) How many monochlorination products would be obtained if all stereoisomers are included?arrow_forwardFill both dashes in the paragraph Compounds containing a phenol group may work as ANTIOXIDANTS to prevent free radical damage. This is accomplished when a free radical (or UV light) encounters a phenol group, turning the phenol group into a radical. However, contrary to typical radical behavior, the structure of the phenol radical can neutralize (or quench) the unpaired electron. Specifically, the phenol structure neutralizes (or quenches) the unpaired radical electron by doing the following: taking the electron and ---------. The correct name (or abbreviation) of an example compound (discussed in the lecture videos) containing a phenol group with antioxidant properties is: ---------.arrow_forwardAlkyl halides undergo nucleophilic substitution and elimination reactions. When the kinetics of the reaction are measured, if the rate of the reaction is found to be dependent only upon the concentration of the alkyl halide the reaction is first order. The substitution reaction is thus termed SN1, and the elimination reaction is termed E1. These reactions are unimolecular and occur in two steps. The first step is rate-limiting and involves the loss of the leaving group to form a carbocation. In the second, fast, step the nucleophile adds to the carbocation in the SN1 reaction or elimination occurs to give an alkene in the E1 reaction. Because the carbocation is planar, the nucleophile can add to either face and therefore racemization is usually observed although solvent effects can influence this somewhat. E1 elimination follows Zaitsev’s rule and typically yields the most substituted alkene as the major product. Conditions which favor the SN1/E1 pathway include the use of a weak…arrow_forward
- Alkyl halides undergo nucleophilic substitution and elimination reactions. When the kinetics of the reaction are measured, if the rate of the reaction is found to be dependent only upon the concentration of the alkyl halide the reaction is first order. The substitution reaction is thus termed SN1, and the elimination reaction is termed E1. These reactions are unimolecular and occur in two steps. The first step is rate-limiting and involves the loss of the leaving group to form a carbocation. In the second, fast, step the nucleophile adds to the carbocation in the SN1 reaction or elimination occurs to give an alkene in the E1 reaction. Because the carbocation is planar, the nucleophile can add to either face and therefore racemization is usually observed although solvent effects can influence this somewhat. E1 elimination follows Zaitsev’s rule and typically yields the most substituted alkene as the major product. Conditions which favor the SN1/E1 pathway include the use of a weak…arrow_forwardFor each of the following, write the major product(s) and then draw out each step in the mechanism using curved arrows. Show ALL lone pair electrons and formal charges. Redraw ALL molecules as to show explicitly ALL bonds being broken or formed. Identify the molecular orbital (HOMO) of the nucleophile and the molecular orbital (LUMO) of electrophile involved in the nucleophilic attack. MO diagrams are not necessary..arrow_forwardThis molecule reacts with HBr to form an alkyl halide. Is this reaction Sn1 or Sn2 and explain your reasoning?arrow_forward
- The following radical-initiated reaction with HBr produces a formal Anti-Markovnikov's rule product. Provide a curved arrow mechanism, making sure to use single headed arrows and drawing all intermediates. Only initiation and propagation steps are needed. Termination does not need to be included. Part of the initiation step is provide for use to generate an OH radical.arrow_forwarda. How many monochlorination products can be obtained from the radical chlorination of methylcyclohexane? Disregard stereoisomers.b. Which product would be obtained in greatest yield? Explain.c. How many monochlorination products would be obtained if all stereoisomers are included?arrow_forwardFill the blank space. Compounds containing a phenol group may work as ANTIOXIDANTS to prevent free radical damage. This is accomplished when a free radical (or UV light) encounters a phenol group, turning the phenol group into a radical. However, contrary to typical radical behavior, the structure of the phenol radical can neutralize (or quench) the unpaired electron. Specifically, the phenol structure neutralizes (or quenches) the unpaired radical electron by doing the following: taking the electron and ---------. The correct name (or abbreviation) of an example compound containing a phenol group with antioxidant properties is: ---------.arrow_forward
- Alkyl halides undergo elimination reactions to produce alkenes by the reacting with strong bases as shown in the following reaction (see image). In general, compounds where the halogen is axial (axial position) are much more reactive than those in which they are in the equatorial position. Taking the above into account: a. Which of the following compounds would give a faster elimination reaction: cis-1-bromo-2-tert-butylcyclohexane or trans-1-bromo-2-tert-butylcyclohexane? Draw the corresponding structures and clearly explain the choice.arrow_forwardWrite the complete reaction mechanism of this reaction including resonance structures of all the productsarrow_forwardpredict the product of each of the following reactions. Mono-halogenated products only for any radical halogenation reactions.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

