
Concept explainers
Name the following
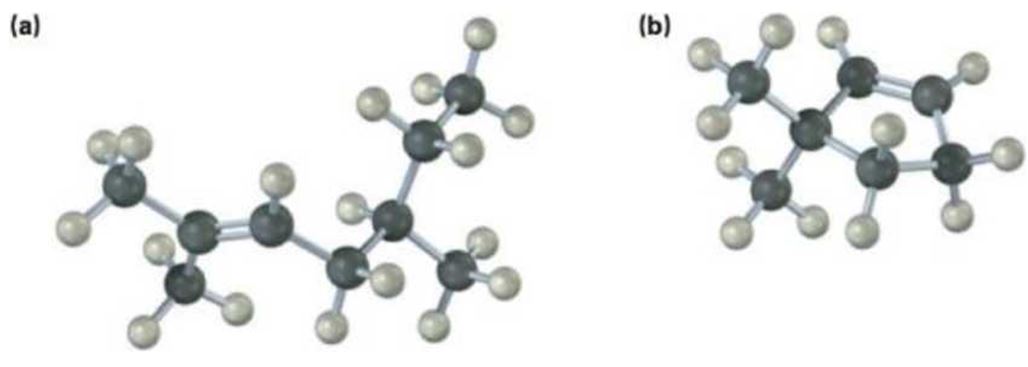
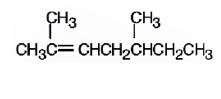
a)
Interpretation:
The name of the alkene shown to be given. The products of its reaction with 1) meta-chloroperoxybenzoic acid, 2) KMnO4 in aqueous acid and 3) O3, followed by Zn in acetic acid, are to be given.
Concept introduction:
When alkenes react with meta-chloroperoxybenzoic acid, oxygen atom adds to the double bond to give epoxides.
Upon treatment with KMnO4 in aqueous acid, if the alkene the double bonded carbon is di-substituted and has no hydrogen it is converted in to a ketone and if the double bonded carbon is mono-substituted and has one hydrogen it is oxidized to a carboxylic acid.
Upon treatment with O3, followed by Zn in acetic acid the double bond is cleaved and each carbon in the double bond gets attached to an oxygen atom to give carbonyl compounds as products. If in the alkene double bonded carbon is di-substituted and has no hydrogen it is converted in to a ketone and if the double bonded carbon is mono-substituted and has one hydrogen it is oxidized to an aldehyde.
To give:
The name of the alkene shown and the products of its reaction with 1) meta-chloroperoxybenzoic acid, 2) KMnO4 in aqueous acid and 3) O3, followed by Zn in acetic acid.
Answer to Problem 22VC
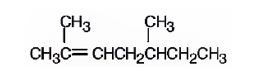
The name of the alkene shown is 2,5-dimethyl-2-heptene.
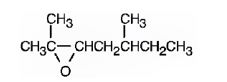
The product formed when it reacts with meta-chloroperoxybenzoic acid is
The products formed when it reacts with KMnO4 in aqueous acid are
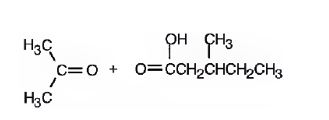
The products formed when it reacts with O3, followed by Zn in acetic acid are
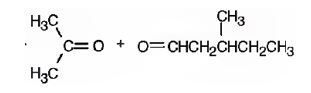
Explanation of Solution
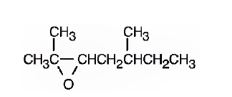
2,5-dimethyl-2-heptene has a double bond between C2&C3. When treated with meta-chloroperoxybenzoic acid, oxygen atom adds to both C2&C3 to give an epoxide.
In 2,5-dimethyl-2-heptene, C2 has no hydrogens attached. So it is oxidized to a ketone.C3 has one hydrogen and is oxidized to a carboxylic acid.
Upon treatment with O3, followed by Zn in acetic acid the double bond between C2&C3 in 2,5-dimethyl-2-heptene is cleaved and each carbon gets attached to an oxygen atom to give a ketone and aldehyde as products.
The name of the alkene shown is 2,5-dimethyl-2-heptene is
The product formed when it reacts with meta-chloroperoxybenzoic acid is
The products formed when it reacts with KMnO4 in aqueous acid are
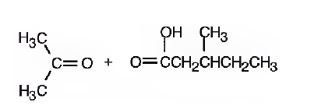
The products formed when it reacts with O3, followed by Zn in acetic acid are
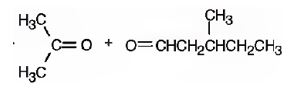
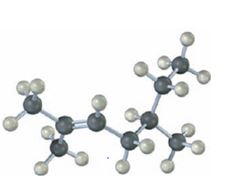
b)
Interpretation:
The name of the alkene shown to be given. The products of its reaction with 1) meta-chloroperoxybenzoic acid, 2) KMnO4 in aqueous acid and 3) O3, followed by Zn in acetic acid, are to be given.
Concept introduction:
When alkenes react with meta-chloroperoxybenzoic acid, oxygen atom adds to the double bond to give epoxides.
Upon treatment with KMnO4 in aqueous acid, if in the alkene double bonded carbon is di-substituted and has no hydrogen it is converted in to a ketone and if the double bonded carbon is mono-substituted and has one hydrogen it is oxidized to a carboxylic acid.
Upon treatment with O3, followed by Zn in acetic acid the double bonded is cleaved and each carbon in the double bond gets attached to an oxygen atom to give carbonyl compounds as products. If in the alkene double bonded carbon is di-substituted and has no hydrogen it is converted in to a ketone and if the double bonded carbon is mono-substituted and has one hydrogen it is oxidized to an aldehyde.
To give:
The name of the alkene shown and the products of its reaction with 1) meta-chloroperoxybenzoic acid, 2) KMnO4 in aqueous acid and 3) O3, followed by Zn in acetic acid.
Answer to Problem 22VC
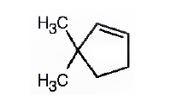
The name of the alkene shown is 3,3-dimethylcyclopentene.
The product formed when it reacts with meta-chloroperoxybenzoic acid is
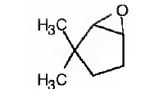
The products formed when it reacts with KMnO4 in aqueous acid are
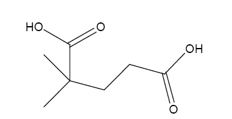
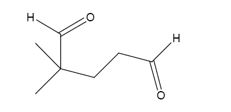
The products formed when it reacts with O3, followed by Zn in acetic acid are
Explanation of Solution
3,3-dimethylcyclopentene has a double bond between C1&C2. When treated with meta-chloroperoxybenzoic acid, oxygen atom adds to both C1&C2 to give an epoxide.
In 3,3-dimethylcyclopentene both C1&C2 have one hydrogen attached to them. So they are oxidized to carboxylic acids when treated with KMnO4.
Upon treatment with O3, followed by Zn in acetic acid the double bond between C1&C2 in 3,3-dimethylcyclopentene is cleaved and each carbon with one hydrogen gets attached to an oxygen atom to yield a dialdehyde as product.
The name of the alkene shown is 3,3-dimethylcyclopentene.

The products formed when it reacts with meta-chloroperoxybenzoic acid are

The products formed when it reacts with KMnO4 in aqueous acid are

The products formed when it reacts with O3, followed by Zn in acetic acid are

Want to see more full solutions like this?
Chapter 8 Solutions
Bundle: Organic Chemistry, Loose-leaf Version, 9th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
- Compound A, C 10H 18O, undergoes reaction with dilute H 2SO 4 at 50 °C to yield a mixture of two alkenes, C 10H 16. The major alkene B, gives only cyclopentanone after ozone treatment followed by reduction with zinc in acetic acid. Which of the following reactions are correct. Can be more than one answerarrow_forwardAmines are converted into alkenes by a two-step process called Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2 reaction when treated with silver oxide as base. Pentylamine, for example, yields 1-pentene. Propose a structure for the intermediate, and explain why it readily undergoes elimination.arrow_forwardBromine reacts with alkenes in methanol according to the equation when this reaction was carried out with 4-tert-butylcyclohexene, only one isomer was formed with the molecular formula C12H23BrO (80% yield). Which of the following is the structure more reasonable for this compound? Explain your reasoning through a corresponding mechanism.arrow_forward
- Name the following alkene and what will be the reaction below with KMnO4 in aqueous acid.arrow_forwardAlkenes can be hydrated to form alcohols by (1) hydroboration followed by oxidation with alkaline hydrogen peroxide and (2) acid-catalyzed hydration. Compare the product formed from each alkene by sequence (1) with those formed from (2). Q.)Cyclopentenearrow_forward1. Deduce the structure of each compound from the information given. All unknowns in this problem have molecular formula C8H12. (a) Upon catalytic hydrogenation, unknown W gives cyclooctane. Ozonolysis of W, followed by reduction with dimethylsulfide, gives octanedioic acid, HOOC—(CH2)6—COOH. Draw the structure of W. (b) Upon catalytic hydrogenation, unknown X gives cyclooctane. Ozonolysis of X, followed by reduction with dimethyl sulfide, gives two equivalents of butanedial, O=CH—CH2CH2—CH=O. Draw the structure of X.arrow_forward
- A chemist allows some pure (2S,3R)-3-bromo-2,3-diphenylpentane to react with a solution of sodium ethoxide(NaOCH2 CH3) in ethanol. The products are two alkenes: A (cis-trans mixture) and B, a single pure isomer. Under the same conditions, the reaction of (2S,3S)-3-bromo-2,3-diphenylpentane gives two alkenes, A (cis-trans mixture) and C. Upon catalytic hydrogenation, all three of these alkenes (A, B, and C) give 2,3-diphenylpentane. Determine the structures of A, B, and C; give equations for their formation; and explain the stereospecificity of these reactions.arrow_forwardA chemist allows some pure (2S,3R)-3-bromo-2,3-diphenylpentane to react with a solution of sodium ethoxide(NaOCH2CH3) in ethanol. The products are two alkenes: A (cis-trans mixture) and B, a single pure isomer. Under thesame conditions, the reaction of (2S,3S)-3-bromo-2,3-diphenylpentane gives two alkenes, A (cis-trans mixture) and C.Upon catalytic hydrogenation, all three of these alkenes (A, B, and C) give 2,3-diphenylpentane. Determine the structuresof A, B, and C; give equations for their formation; and explain the stereospecificity of these reactionsarrow_forwardEach of the following alcohols has been subjected to acid-catalyzed dehydration and yields a mixture of two isomeric alkenes. Identify the two alkenes in each case, and predict which one is the major product.arrow_forward
- Alkenes can be hydrated to form alcohols by (1) hydroboration followed by oxidation with alkaline hydrogen peroxide and (2) acid-catalyzed hydration. Compare the product formed from each alkene by sequence (1) with those formed from (2). Q.) cis-2-Butenearrow_forward4. Compound A has the formula C 8H 8. It reacts rapidly with KMnO 4 to give CO 2 and a carboxylic acid, B (C 7H 6O 2), but reacts with only 1 molar equivalent of H 2 on catalytic hydrogenation over a palladium catalyst. On hydrogenation under conditions that reduce aromatic rings, 4, equivalents of H 2 are taken up and hydrocarbon C (C 8H 16) is produced. What are the structures of A, B, and C.arrow_forwardA certain compound of molecular formula C19H38 was isolated from fish oil and from plankton. On hydrogenation it gave 2,6,10,14-tetramethylpentadecane. Ozonolysis gave (CH3)2C O and a 16-carbon aldehyde. What is the structure of the natural product? What is the structure of the aldehyde?arrow_forward
