
a)
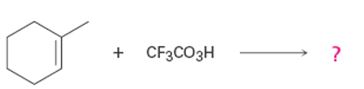
Interpretation:
Product formed in the reaction with the mechanism of its formation is to be given.
Concept introduction:
To give:
Product formed in the reaction with the mechanism of its formation.
b)
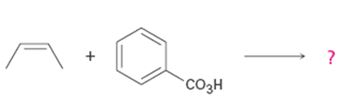
Interpretation:
Product formed in the reaction with the mechanism of itsformation is to be given.
Concept introduction:
Alkenes are oxidized to give epoxides on treatment with peroxyacids. The addition of oxygen to the double bond occurs with syn stereochemistry- both C-O bonds are formed on the same face of the double bond. The oxygen atom farthest from the carbonyl group is transferred to the double bond.
To give:
Product formed in the reaction with the mechanism of its formation.
Trending nowThis is a popular solution!

Chapter 8 Solutions
Bundle: Organic Chemistry, Loose-leaf Version, 9th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
- Plesse show the mechanism of all the reactions and show the profucts stereoisomericallyarrow_forwardPredict the products of conjugate (Michael) additions, and show how to use thesereactions in syntheses. Show the general mechanism of the Robinson annulation,and use it to form cyclohexenone ring systems.arrow_forwardTreatment of (CH3)2CHCH(OH)CH2CH3 with TsOH affords two products (M and N) with molecular formula C6H12. The 1H NMR spectra of M and N are given below. Propose structures for M and N, and draw a mechanism to explain their formation.arrow_forward
