
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 20P
How many
(a)

(b)

(c)
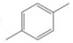
(d)
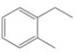
(e)
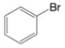
(f)
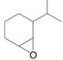
(g)
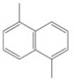
(h)
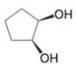
(i)
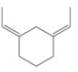
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
How many 1H NMR signals would you expect for each compound?
Answer the following questions about each of the hydroxy ketones: 1-hydroxybutan-2-one (A) and 4-hydroxybutan-2-one (B).
a.) How many signals are observed in the 1H NMR spectrum?b.) Give the splitting observed for each type of proton as well as its approximate chemical shift.
Draw the structure for a compound with the formula C3H6Br2 that matches the following 1H-NMR spectrum:
Chapter 9 Solutions
Organic Chemistry
Ch. 9 - Prob. 1PPCh. 9 - PRACTICE PROBLEM 9.2 What compound with molecular...Ch. 9 - PRACTICE PROBLEM 9.3
Using the method of Section...Ch. 9 - PRACTICE PROBLEM 9.4 How many signals would each...Ch. 9 - Prob. 5PPCh. 9 - Prob. 6PPCh. 9 - PRACTICE PROBLEM 9.7
The relative chemical shifts...Ch. 9 - Prob. 8PPCh. 9 - PRACTICE PROBLEM 9.9 Propose a structure for...Ch. 9 - PRACTICE PROBLEM 9.10
What is the dihedral angle...
Ch. 9 - PRACTICE PROBLEM 9.11 Draw the most stable chair...Ch. 9 - Prob. 12PPCh. 9 - PRACTICE PROBLEM 9.13 How many signals would you...Ch. 9 - Prob. 14PPCh. 9 - Prob. 15PPCh. 9 - Prob. 16PPCh. 9 - Prob. 17PPCh. 9 - PRACTICE PROBLEM 9.18
What are the expected ratios...Ch. 9 - Prob. 19PPCh. 9 - How many 1H NMR signals (not peaks) would you...Ch. 9 - How many 13C NMR signals would you predict for...Ch. 9 - Prob. 22PCh. 9 - Prob. 23PCh. 9 - Prob. 24PCh. 9 - Compound Q has the molecular formula C7H8. The...Ch. 9 - 9.26 Explain in detail how you would distinguish...Ch. 9 - Compound S (C8H16) reacts with one mole of bromine...Ch. 9 - A compound with molecular formula C4H8O has a...Ch. 9 - In the mass spectrum of 2, 6-dimethyl-4-heptanol...Ch. 9 - Prob. 30PCh. 9 - What are the masses and structures of the ions...Ch. 9 - Prob. 32PCh. 9 - Ethyl bromide and methoxybenzene (shown below)...Ch. 9 - 9.34 The homologous series of primary amines, ,...Ch. 9 - Propose a structure that is consistent with each...Ch. 9 - 9.36 Propose structures for compounds E and F....Ch. 9 - 9.37 Use the NMR and IR data below to propose a...Ch. 9 - 9.38 When dissolved in , a compound (K) with the...Ch. 9 - Compound T (C5H8O) has a strong IR absorption band...Ch. 9 - Prob. 40PCh. 9 - Deduce the structure of the compound that gives...Ch. 9 - Deduce the structure of the compound that gives...Ch. 9 - The 1H NMR spectrum of a solution of 1,...Ch. 9 - Acetic acid has a mass spectrum showing a...Ch. 9 - The 1H NMR peak for the hydroxyl proton of...Ch. 9 - The 1H NMR study of DMF (N, N-dimethylformamide)...Ch. 9 - 9.48 The mass spectra of many benzene derivatives...Ch. 9 - Prob. 49PCh. 9 - 1. Given the following information, elucidate the...Ch. 9 - Two compounds with the molecular formula C5H10O...
Additional Science Textbook Solutions
Find more solutions based on key concepts
1.6 Read the labels on products used to wash your dishes. What are the names of some chemicals contained in tho...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
The molar concentration of phosgene at equilibrium and Keqfor the system needs to be calculated. Concept Introd...
Chemistry: Matter and Change
For each of the following, (i) give the systematic name of the compound and specify the oxidation state of the ...
General Chemistry: Atoms First
10.71 Identify each of the following as an acid or a base: (10.1)
H2SO4
RbOH
Ca(OH)2
HI
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Which of the following are not acids? CH3COOH CO2 HNO2 HCOOH CCl4
Essential Organic Chemistry (3rd Edition)
Balance the following equations by inspection. a. P2H2PH2+P4 b. P4+CI2PCI2 c. FeCI2+H2SFe2S3+HCI d. Mg2N2+H2OMg...
General Chemistry: Principles and Modern Applications (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The compound with the closed formula C4H9Br gives the following 1H NMR data. Write the clear formula of the compound with reasons. 1.0 ppm (triplet, 3 H); 1.7 ppm (dublet, 3H); 1.8 ppm (multiplet, 2H); 4.2 ppm (multiplet, 1H)arrow_forwardHow many signals would you expect to see for the indicated hydrogens in the following compound’s 1H NMR spectrum?arrow_forwardhow many signals would appear in 1H NMR spectrum of the compounds given belowarrow_forward
- Which of the following compounds has two triplets and one singlet in its 1H NMR spectrum?arrow_forward1. How could you use chemical shift and integration data to distinguish between each pair of compounds, if the 1H NMR spectrum of each compound contains only singletsarrow_forwardWhich of (a)-(d) indicates the correct order of chemical shifts of the four carbons in the 13C-NMR spectrum of the following compound.arrow_forward
- How many H-NMR signals would you expect to see for this compound?arrow_forwardDraw an isomer of dichlorocyclopropane that gives an 1H NMR spectrum a. with one signal. b. with two signals. c. with three signals.arrow_forwardWhich set of underlined hydrogens has its 1H NMR signal at a higher frequency?arrow_forward
- How many proton NMR signals would you expect from the isomers below? Also indicate their approximate chemical shift (relative position on the chart). Can you explain how to find the proton NMR signal and their chemical shift?arrow_forward1) Propose the structures for the following 1H and 13C NMR spectrum. b) Molecular Formula: C8H8O3 1H-NMR, CDCl3 Solvent, Molecular Formula: C8H8O3 13C-NMR, CDCl3 Solvent, Molecular Formula: C8H8O3 in the picturesarrow_forward(a) How many 1H NMR signals does each of the following compounds exhibit? (b) How many 13C NMR signals does each compound exhibit?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY