
Concept explainers
Deduce the structure of the compound that gives the following
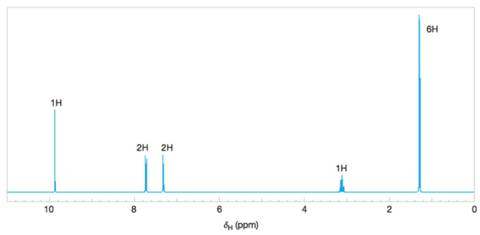
Figure 9.43 The
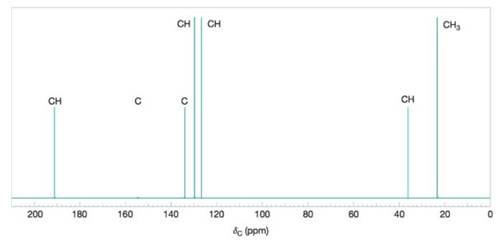
Figure 9.44 A simulated broadband proton-decoupled
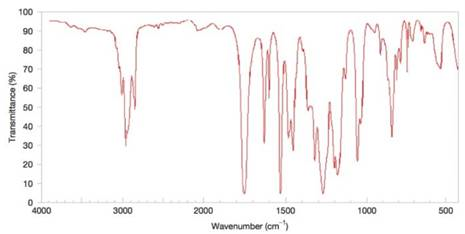
Figure 9.45 The IR spectrum for Problem 9.41. (SDBS, National Institute of Advanced Industrial Science and Technology)
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Chemistry: The Molecular Nature of Matter
Chemistry & Chemical Reactivity
Living By Chemistry: First Edition Textbook
Introductory Chemistry (5th Edition) (Standalone Book)
Chemistry For Changing Times (14th Edition)
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
- Give the number of peaks that will show up in 1H NMR spectrum of the 2 compounds belowarrow_forwardPropose a structure for your product and justify your assignment based on its melting point and its 1H NMR spectrum. The analysis of the 1H NMR spectrum should include a detailed assignment of each of the NMR resonances to the protons found in your structure. NMR analysis should include reasoning used to exclude other aldehydes and ketones from your structural assignment. For example, no signals were observed between 0-4 ppm indicating no H atoms bound to sp3 hybridized carbons. It can be concluded from this observation that no ketone or aldehyde that would retain a H bound to sp3 hybridized carbons were used as starting materials.arrow_forwardanalyse high resolution proton NMR spectrum and suggest a compound and write its name. Also write down which parts of the molecule belongs to each peak.(max character:60000)arrow_forward
- Provide the structure of the molecule that would give rise to these spectra. Include degree of unsaturation, functional groups present, assignment of 1H and 13C NMR peaks by labeling each peak with specific atoms in the structure (a,b,c,d) and show which atoms give rise at which peaksarrow_forwardFor the compounds below give the 1H NMR data (chemical shift, integration and multiplicity) I appreciate the help.arrow_forwardThe mass spectrum of tert-butylamine follows shows an intense base peak at m>z 58, and very little else. Use a diagram toshow the cleavage that accounts for the base peak. Suggest why no molecular ion is visible in this spectrum.1arrow_forward
- Propose structures that are consistent with the following spectra. (Integral ratios are given from left to right across the spectrum.) a. The 1H NMR spectrum of a compound with molecular formula C4H10O2 has two singlets with integral ratios of 2 : 3. b. The 1H NMR spectrum of a compound with molecular formula C6H10O2 has two singlets with integral ratios of 2 : 3. c. The 1H NMR spectrum of a compound with molecular formula C8H6O2 has two singlets with integral ratios of 1 : 2.arrow_forwardA two-step synthesis of virstatin reactions are given. Give the yield and % yield of each synthesis. Make a labeled drawing of the equivalent hydrogens for each product. Using the NMR drawings given for each structure, describe how the hydrogens are seen on the NMR by chemical shift, integration, and splitting. Make sure your NMR matches the structure of the product you are proposing. For example: “the triplet at 4ppm, integrating to 2, represents the methylene group (Ha) attached to the nitrogen, as it deshielded by the electronegative element and is split by the neighboring methylene group. If you did not make the intended product in step one of the two step synthesis, explain why it did not matter in regards to further reacting the products to form virstatin. Data Mass of 1,8-naphthalimide used: 0.2528g Mass of potassium carbonate used: 0.2624g Recovered virstatin ethyl ester: 0.2602gMass of virstatin ethyl ester used in step 2: 0.1066g Mass of recovered virstatin: 0.0035g…arrow_forwardFor the following compound: C3H5BrO2 (Molecular Weight = 152) -Calculate the degrees of unsaturation -Use the IR and NMR to determine the structure for your compound -All 1H NMR peaks (number of signals, chemical shift, integration and splitting)-All 13C NMR peaks (number of signals and chemical shift)-All IR peaks that are important (no need to look into the fingerprint region!)-Your proposed structure of the compound (C3H5BrO2) and how it matches with the spectra (images attached)arrow_forward
- Give the structure for two isomers of molecular formula C4H10O which are consistent with the ^1H-NMR spectra shown below.arrow_forwardThe mass spectrum and 13C NMR spectrum of a hydrocarbon are shown. Propose a structure for this hydrocarbon, and explain the spectral data.arrow_forwardThe infrared spectrum of the compound with the mass spectrum shown below lacks any significant absorption above 3000 cm-1. There is a prominent peak near 1740 cm-1 and another strong peak near 1200 cm-1. Propose a structure consistent with the data.arrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning



