
Chemistry In Context
9th Edition
ISBN: 9781259638145
Author: Fahlman, Bradley D., Purvis-roberts, Kathleen, Kirk, John S., Bentley, Anne K., Daubenmire, Patrick L., ELLIS, Jamie P., Mury, Michael T., American Chemical Society
Publisher: Mcgraw-hill Education,
expand_more
expand_more
format_list_bulleted
Question
Chapter 9, Problem 8Q
Interpretation Introduction
Interpretation:
The reason why repeating head–to–tail arrangement is not possible in ethylene has to be identified.
Concept Introduction:
Monomer: A molecule is considered as monomer when this molecule bonds with another identical molecule which results to form polymer.
Terpenes are made by joining five-carbon units, usually in a head to tail-fashion.
Isoprene unit:
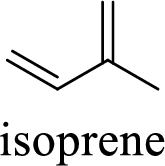
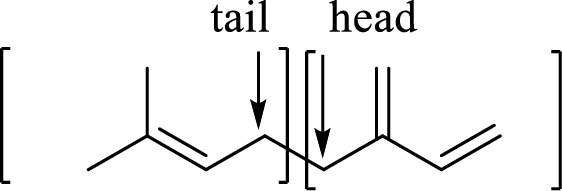
Branched end of isoprene – Head
Unbranched end of isoprene - Tail
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is polymer condensation?
What intermolecular force/s of attraction is not involved in producing a biodegradable plastic bag (polyethylene and polyvinyl alcohol)?
How does a homopolymer differ from a copolymer?
Chapter 9 Solutions
Chemistry In Context
Ch. 9.1 - Scientific Practices Tennis Anyone? Examine this...Ch. 9.3 - Prob. 9.2YTCh. 9.3 - Prob. 9.3YTCh. 9.4 - Prob. 9.4YTCh. 9.4 - Prob. 9.5YTCh. 9.4 - Prob. 9.6YTCh. 9.4 - Prob. 9.7YTCh. 9.4 - Prob. 9.8YTCh. 9.4 - Prob. 9.9YTCh. 9.5 - Prob. 9.10YT
Ch. 9.5 - Skill Building Benzene and Phenyl The difference...Ch. 9.5 - Prob. 9.13YTCh. 9.5 - Skill Building Polystyrene Possibilities Show the...Ch. 9.6 - Skill Building Esters and Polyesters You have seen...Ch. 9.6 - Prob. 9.16YTCh. 9.7 - Skill Building Kevlar Kevlar is a polyamide used...Ch. 9.8 - Prob. 9.20YTCh. 9.8 - Your Turn 9.22 Skill Building Burning a Plastic...Ch. 9.8 - Your Turn 9.23 Scientific Practices Landfill...Ch. 9.9 - Examine the values in Table 9.4 from the American...Ch. 9.9 - Prob. 9.25YTCh. 9.9 - Prob. 9.26YTCh. 9.9 - Prob. 9.28YTCh. 9.10 - Skill Building The Chemistry of PLA We dont show...Ch. 9.11 - Your Turn 9.31 Scientific Practices Glass or...Ch. 9.11 - Prob. 9.32YTCh. 9.11 - Skill Building Meet DEHP DEHP belongs to a common...Ch. 9 - Prob. 1QCh. 9 - Prob. 2QCh. 9 - Prob. 3QCh. 9 - Prob. 4QCh. 9 - Prob. 5QCh. 9 - Prob. 6QCh. 9 - Prob. 7QCh. 9 - Prob. 8QCh. 9 - Prob. 9QCh. 9 - Prob. 10QCh. 9 - Prob. 11QCh. 9 - Prob. 12QCh. 9 - Prob. 13QCh. 9 - Prob. 14QCh. 9 - Prob. 15QCh. 9 - Prob. 16QCh. 9 - Prob. 17QCh. 9 - Prob. 18QCh. 9 - Prob. 19QCh. 9 - Prob. 20QCh. 9 - Prob. 21QCh. 9 - Prob. 22QCh. 9 - Prob. 23QCh. 9 - Prob. 24QCh. 9 - Prob. 25QCh. 9 - Prob. 26QCh. 9 - Prob. 27QCh. 9 - Prob. 28QCh. 9 - Prob. 29QCh. 9 - Prob. 30QCh. 9 - Prob. 31QCh. 9 - Prob. 32QCh. 9 - Prob. 33QCh. 9 - Prob. 34QCh. 9 - Prob. 35QCh. 9 - Prob. 36QCh. 9 - Prob. 37QCh. 9 - Prob. 38QCh. 9 - Prob. 39QCh. 9 - Prob. 40QCh. 9 - Prob. 41QCh. 9 - Prob. 42QCh. 9 - Prob. 43QCh. 9 - Prob. 44QCh. 9 - Prob. 45QCh. 9 - Prob. 46QCh. 9 - Prob. 47QCh. 9 - Prob. 48QCh. 9 - Prob. 49QCh. 9 - Prob. 50QCh. 9 - Prob. 51QCh. 9 - Prob. 52QCh. 9 - Prob. 53QCh. 9 - Prob. 54QCh. 9 - Prob. 55QCh. 9 - Prob. 56QCh. 9 - Prob. 57QCh. 9 - Prob. 58QCh. 9 - Prob. 59Q
Knowledge Booster
Similar questions
- .Explain the term polymerization with two examples.arrow_forwardStep-growth, or condensation, polymers are formed from monomers that have more than one functional group. Consider the monomers oxalyl chloride and resorcinol, shown below. Draw the structure for two repeat units of the polymer that forms from these monomersarrow_forwardWhat is trichlorocatechol used for in the pulp and paper industry?arrow_forward
- what is the monomer polyethene?arrow_forwardWhat is the drawing of the monomers used to make sodium polyacrylate polymer chains? only the monomerarrow_forwardWhat types of intermolecular forces are present in liquid and solid samples of the organic compound given as a line structure below? What types are not present?arrow_forward
- Is polystyrene biodegradable or compostable and why??arrow_forwardWhy is sulfur mustard comparatively less permeable to urethane polymer than polymers like polyethylene. Will a polyethylene gas mask be effective against sulfur mustard?arrow_forwardSilk is an example of a natural polymer. Name three properties that make silk desirable.Which synthetic polymer has a chemical structure modeled after silk?arrow_forward
- Provide two examples of thermoresponsive polymer and its applications in modern technologiesarrow_forwardDifferentiate between thermoplastic and thermosetting polymers. Give one example of each.arrow_forwardSOLUTIONS TO OVERCOME PLASTIC POLLUTION - Briefly explain how to reuse reduce and recycle plastic to prevent plastic pollution in the environment.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning