
Concept explainers
Ethylbenzene is converted to styrene in the catalytic dehydrogenation reaction C«H10(g) -
A flowchart of a simplified version of the commercial process is shown here.
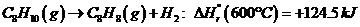
Fresh and recycled liquid ethylbenzene combine and are heated from 25°C to 500°C (A). and the heated ethylbenzene is mixed adiabatically with steam at 700°C (B) to produce the feed to the reactor at 600°C. (The steam suppresses undesired side reactions and removes carbon deposited on the catalyst surface.) A once-through conversion of 35% is achieved in the reactor (C), and the products emerge at 560°C. The product stream is cooled to 25°C (D), condensing essentially all of the water, ethylbenzene, and styrene and allowing hydrogen to pass out as a recoverable by-product of the process.
The water and hydrocarbon liquids are immiscible and are separated in a settling tank decanter (£). The water is vaporized and healed (F) to produce the steam that mixes with the ethylbenzene feed to the reactor. The hydrocarbon stream leaving the decanter is fed to a distillation tower(C) (actually, a series of towers), which separates the mixture into essentially pure styrene and ethylbenzene, each at 25°C after cooling and condensation steps have been carried out. The ethylbenzene is recycled to the reactor preheater, and the styrene is taken off as a product.
- On a basis of 100 kg/h styrene produced, calculate the required fresh ethylbenzene feed rale, the flow rate of recycled ethylbenzene, and the circulation rate of water, all in mol/h. (Assume P = 1 atm.)
- Calculate the required rates of heat input or withdrawal in kJ/h for the ethylbenzene preheater (A), steam generator (F), and reactor (C).
Physical Property Data
Learn your wayIncludes step-by-step video

Chapter 9 Solutions
Elementary Principles of Chemical Processes, Binder Ready Version
Additional Engineering Textbook Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Process Dynamics and Control, 4e
Digital Fundamentals (11th Edition)
Foundation Design: Principles and Practices (3rd Edition)
Experiencing MIS
Structural Analysis (10th Edition)
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





