
Concept explainers
(a)
Interpretation:
The complete, detailed mechanism for the reaction is to be provided. The stereochemistry of the products is to be predicted where appropriate. If the reaction yields exclusively one product or a mixture of products, then the major product is to be determined.
Concept introduction:
For the prediction of the outcome of the
Answer to Problem 9.64P
The complete, detailed mechanism for the reaction is shown below:
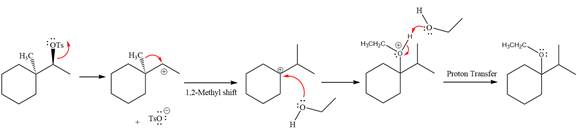
Explanation of Solution
The given reaction is
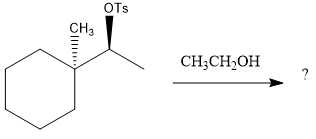
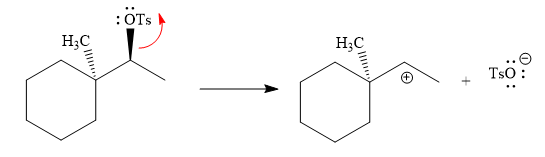
The absolute configuration of the substrate is S. This is solvolysis reaction because ethanol acts as a nucleophile and a solvent. The leaving group,
The attacking species
The loss of the leaving group,
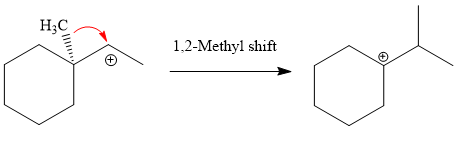
A tertiary carbocation is highest stability than a secondary and primary carbocation. Next,
Now, the weak nucleophile,
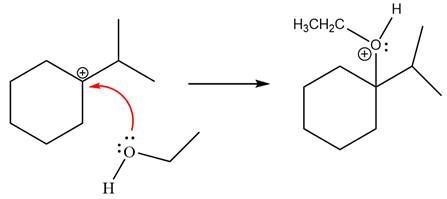
Finally, deprotonation of the
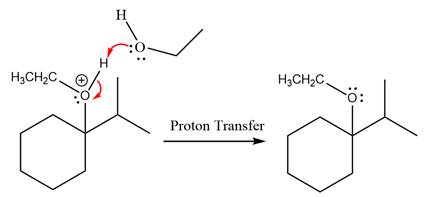
The detailed, complete mechanism is as follows:
The outcome of the given reaction is predicted by considering factors like the nature of the leaving group, substrate, the strength of the reagent used, solvent, and temperature.
(b)
Interpretation:
The complete, detailed mechanism for the reaction is to be provided. The stereochemistry of the products is to be predicted where appropriate. If the reaction yields exclusively one product or a mixture of products, then the major product is to be determined.
Concept introduction:
For the prediction of the outcome of the
Answer to Problem 9.64P
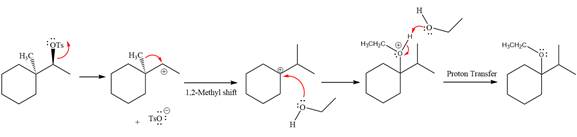
The complete, detailed mechanism for the reaction is shown below:
The stereochemistry of the product is R configuration.
Explanation of Solution
The given reaction is
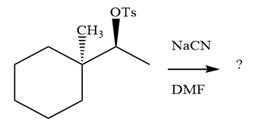
The absolute configuration of the substrate is S. The leaving group,
The attacking species
The carbon atom attached to the leaving group is a chiral center.
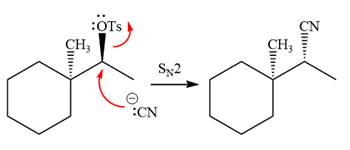
The stereochemistry of the product is R configuration.
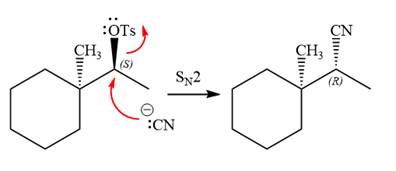
The outcome of the given reaction is predicted by considering factors like the nature of the leaving group, substrate, the strength of the reagent used, solvent, and temperature.
(c)
Interpretation:
The complete, detailed mechanism for the reaction is to be provided. The stereochemistry of the products is to be predicted where appropriate. If the reaction yields exclusively one product or a mixture of products, then the major product is to be determined.
Concept introduction:
For the prediction of the outcome of the
Answer to Problem 9.64P
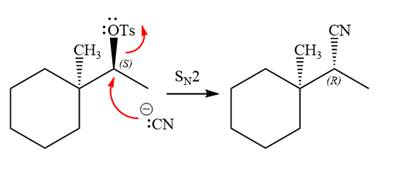
The complete, detailed mechanism for the reaction is shown below:
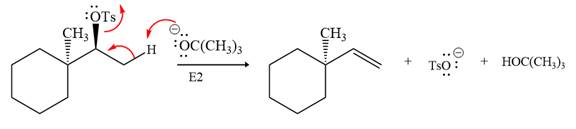
Explanation of Solution
The given reaction is
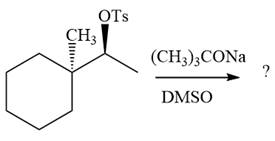
The absolute configuration of the substrate is S. The leaving group,
The attacking species
In the substrate, the alpha C atom has one H atom attached to it. Since

The outcome of the given reaction is predicted by considering factors like the nature of the leaving group, substrate, the strength of the reagent used, solvent, and temperature.
(d)
Interpretation:
The complete, detailed mechanism for the reaction is to be provided. The stereochemistry of the products is to be predicted where appropriate. If the reaction yields exclusively one product or a mixture of products, then the major product is to be determined.
Concept introduction:
For the prediction of the outcome of the
Answer to Problem 9.64P
The complete, detailed mechanism for the reaction is shown below:
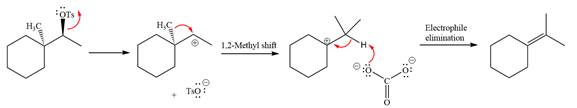
Explanation of Solution
The given reaction is
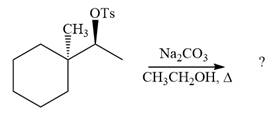
The absolute configuration of the substrate is S. The leaving group,
The attacking species
The loss of the leaving group,
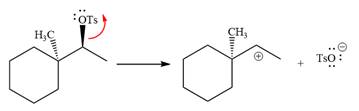
A tertiary carbocation is greatest stability than a secondary and primary carbocation. Next,
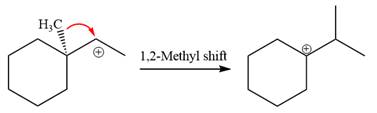
Now, the weak nucleophile
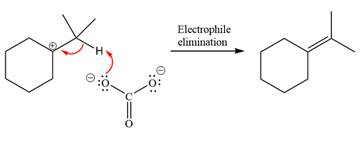
The detailed, complete mechanism is as follows:
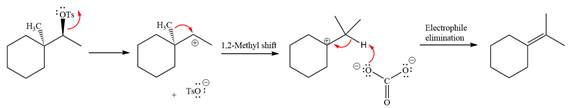
The outcome of the given reaction is predicted by considering factors like the nature of the leaving group, substrate, the strength of the reagent used, solvent, and temperature.
(e)
Interpretation:
The complete, detailed mechanism for the reaction is to be provided. The stereochemistry of the products is to be predicted where appropriate. If the reaction yields exclusively one product or a mixture of products, then the major product is to be determined.
Concept introduction:
For the prediction of the outcome of the
Answer to Problem 9.64P
The complete, detailed mechanism for the reaction is shown below:
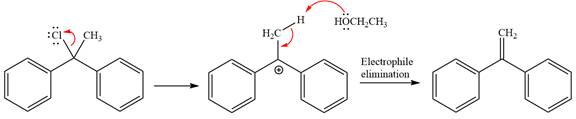
Explanation of Solution
The given reaction is
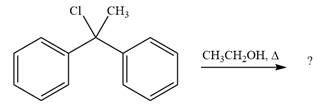
This is a solvolysis reaction because ethanol acts as a nucleophile and the solvent. The leaving group,
The attacking species
The loss of the leaving group,
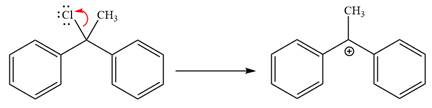
Now, the weak nucleophile
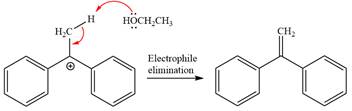
The detailed, complete mechanism is as follows:
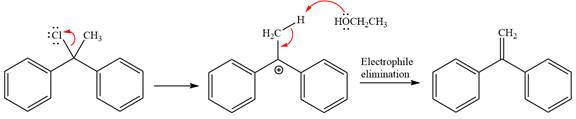
The outcome of the given reaction is predicted by considering factors like the nature of the leaving group, substrate, the strength of the reagent used, solvent, and temperature.
(f)
Interpretation:
The complete, detailed mechanism for the reaction is to be provided. The stereochemistry of the products is to be predicted where appropriate. If the reaction yields exclusively one product or a mixture of products, then the major product is to be determined.
Concept introduction:
For the prediction of the outcome of the
Answer to Problem 9.64P
The complete, detailed mechanism for the reaction is shown below:
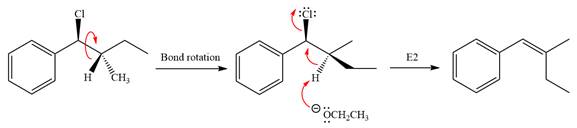
Explanation of Solution
The given reaction is
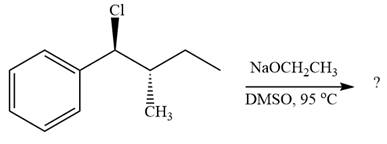
The leaving group,
The attacking species
In the substrate, only one H atom can be eliminated. The leaving group and H atom are on same side.
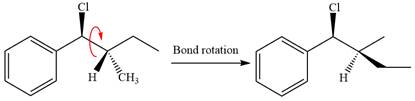
The base deprotonates a hydrogen atom from the substrate and to yield the most substituted alkene as the product.
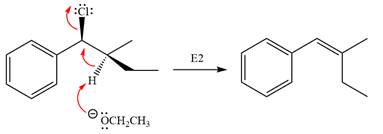
The detailed, complete mechanism is as follows:
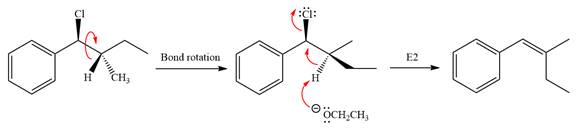
The outcome of the given reaction is predicted by considering factors like the nature of the leaving group, substrate, the strength of the reagent used, solvent, and temperature.
Want to see more full solutions like this?
Chapter 9 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- provide the major product(s) for each of the following reactions. also, provide the mechanism for the last two reactionsarrow_forwardDraw a detailed mechanism of the following reactions and determine the major product:arrow_forwardGiven the information below, write out a reasonable mechanism for the reaction. Where not provided, also fill in the major product(s) of the reaction: Show all arrowsarrow_forward
- Draw the complete, detailed mechanism for each of the following reactions and predict the major products.arrow_forwardDraw the mechanism and the major organic product for each of the following reactions. Please show all work with arrowsarrow_forwardprovide the detailed mechanism for the given reactions. Do not skip any steparrow_forward
- Propose a mechanism for the reaction shown here, which takes place under conditions that favor anarrow_forwardWhat is the name of the reaction and what is the product? provide the detailed mechanism pleasearrow_forwardprovide the major product(s) and full mechanism for the following reactionsarrow_forward
- Determine the major product of each reaction in Problem and draw the complete, detailed mechanism. Pay attention to stereochemistry where appropriate.arrow_forwardPlease draw detailed mechanism of this reaction. Picture are attached:arrow_forwardThe reaction shown here is a halosulfonation, which is a useful variation of the sulfonation reaction. Draw the complete mechanism for this reaction.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





