
Concept explainers
(a)
Interpretation:
It is to be determined whether the given
Concept introduction:
Competing reactions can take place in kinetic or
Reactions that tend to take place under thermodynamic control are the ones in which a more stable but not necessarily the major product is formed.
Reversible reactions tend to take place under thermodynamic control, while irreversible reactions tend to take place under kinetic control.
Reactions like
The charge stability decides if the products are more stable than the reactants or vice versa. A reaction is irreversible if it’s
Answer to Problem 9.72P
The given reaction is irreversible.
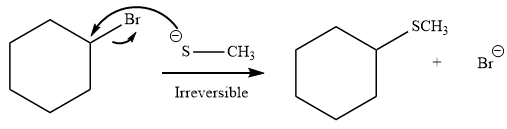
Explanation of Solution
The given reaction is:
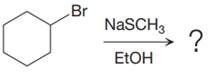
It is mentioned that the reaction would follow the
The species
As the reaction is irreversible it would take place under kinetic control.
The charge stability decides if the products are more stable than the reactants or vice versa.
(b)
Interpretation:
It is to be determined whether the given
Concept introduction:
Competing reactions can take place in kinetic or thermodynamic control. Reactions that tend to take place under the kinetic control are the ones in which the major product is the one that forms the fastest.
Reactions that tend to take place under thermodynamic control are the ones in which a more stable but not necessarily the major product is formed.
Reversible reactions tend to take place under thermodynamic control, while irreversible reactions tend to take place under kinetic control.
Reactions like
The charge stability decides if the products are more stable than the reactants or vice versa. A reaction is irreversible if it’s
Answer to Problem 9.72P
The given reaction is reversible.
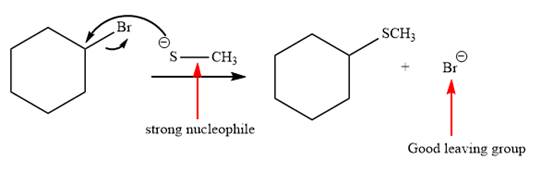
Explanation of Solution
The given reaction is:
It is mentioned that the reaction would follow the
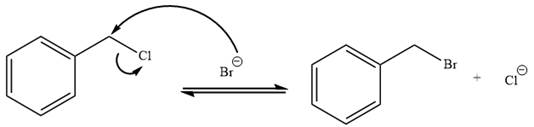
The charge stability decides if the products are more stable than the reactants or vice versa. The charged species on the left side is
This makes the reaction faster in the reverse direction than in the forward direction under standard conditions. Thus, this reaction is reversible.
The charge stability decides if the products are more stable than the reactants or vice versa.
(c)
Interpretation:
It is to be determined whether the given
Concept introduction:
Competing reactions can take place in kinetic or thermodynamic control. Reactions that tend to take place under the kinetic control are the ones in which the major product is the one that forms the fastest.
Reactions that tend to take place under thermodynamic control are the ones in which a more stable but not necessarily the major product is formed.
Reversible reactions tend to take place under thermodynamic control, while irreversible reactions tend to take place under kinetic control.
Reactions like
The charge stability decides if the products are more stable than the reactants or vice versa. A reaction is irreversible if it’s
Answer to Problem 9.72P
The given reaction is irreversible.
Explanation of Solution
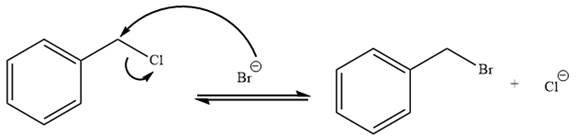
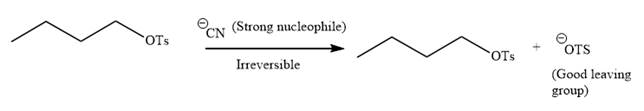
The given reaction is:
It is mentioned that the reaction would follow the
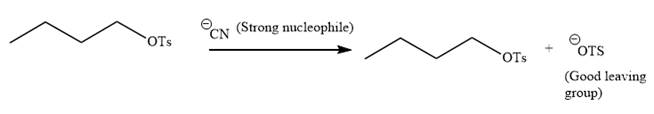
In the above reaction,
The charge stability decides if the products are more stable than the reactants or vice versa. The charged species on the left side is
Thus,
As the reaction is irreversible it would take place under kinetic control.
The charge stability decides if the products are more stable than the reactants or vice versa.
Want to see more full solutions like this?
Chapter 9 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- Explain how you can tell from the energy diagram that the reaction with the catalyst in Fig. 8.4 isfaster than the reaction without the catalyst.arrow_forwardA student proposes the following reaction mechanism for the reaction in Model 6. Which step inthis mechanism is least favorable? Explain your reasoning.arrow_forwardFor each set of reactions, circle the mechanism (SN2 vs SN1), draw the main organic substitution/elimination product (for each reaction draw the product, though in some cases it may be equivalent) and indicate which reaction occurs at the faster rate.arrow_forward
- Explain why the following reaction is favorable or unfavorable.arrow_forwardhey could someone help me rank these compounds in order of their reactivity with an SN1 reaction? I’m not sure how to do it, thanks!arrow_forwardHow will the rate of each of the following SN2 reactions change if it is carried out in a more polar solvent?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
