
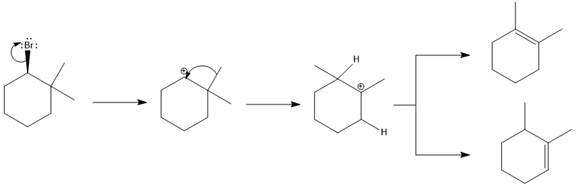
Concept explainers
(a)
Interpretation:
For the given substrate, when
Concept introduction:
As the number of alkyl groups on the carbon bonded to the leaving group increases, the
Answer to Problem 9.69P
The major E1 product for the given reaction is shown below:
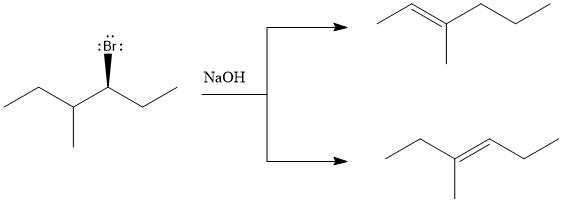
Explanation of Solution
Elimination usually takes place so as to produce the most stable, i.e., highly alkyl substituted, alkene.
The possible E1 products are obtained by eliminating the leaving group and a proton on carbon adjacent to the one bonded to the leaving group. Here the
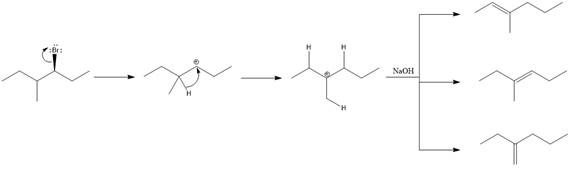
The first and second alkene product is highly substituted than the third alkene product. Therefore, the first and second alkene products are more stable. The base in this reaction is OH, which is not bulky, so the first and second products are the major products.

From the stability of the product formed, the major E1 product is drawn.
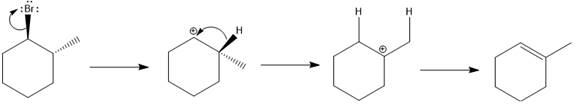
(b)
Interpretation:
For the given substrate, when
Concept introduction:
As the number of alkyl groups on the carbon bonded to the leaving group increases, the rate of E1 reaction increases. Each additional alkyl group, which is electron donating, stabilizes the carbocation intermediate produced and thus helps the leaving group leave. The
Answer to Problem 9.69P
The major E1 product for the given reaction is shown below:
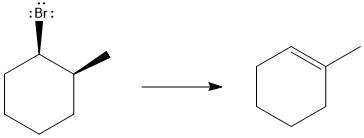
Explanation of Solution
Elimination usually takes place so as to produce the most stable, i.e., highly alkyl substituted, alkene.
The possible E1 products are obtained by eliminating the leaving group and a proton on carbon adjacent to the one bonded to the leaving group. Here the
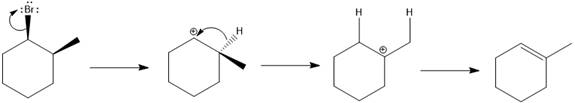
From the stability of the product formed, the major E1 product is drawn.
(c)
Interpretation:
For the given substrate, when
Concept introduction:
As the number of alkyl groups on the carbon bonded to the leaving group increases, the rate of E1 reaction increases. Each additional alkyl group, which is electron donating, stabilizes the carbocation intermediate produced and thus helps the leaving group leave. The
Answer to Problem 9.69P
The major E1 product for the given reaction is shown below:
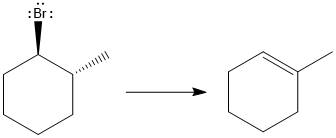
Explanation of Solution
Elimination usually takes place so as to produce the most stable, i.e., highly alkyl substituted, alkene.
The possible E1 products are obtained by eliminating the leaving group and a proton on carbon adjacent to the one bonded to the leaving group. Here the
From the stability of the product formed, the major E1 product is drawn.
(d)
Interpretation:
For the given substrate, when
Concept introduction:
As the number of alkyl groups on the carbon bonded to the leaving group increases, the rate of E1 reaction increases. Each additional alkyl group, which is electron donating, stabilizes the carbocation intermediate produced and thus helps the leaving group leave. The
Answer to Problem 9.69P
The major E1 product for the given reaction is shown below:
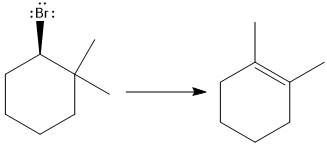
Explanation of Solution
Elimination usually takes place so as to produce the most stable that means highly alkyl substituted alkene.
The possible E1 products are obtained by eliminating the leaving group and a proton on the carbon adjacent to the one bonded to the leaving group. Here the
The first alkene product is highly substituted than the second alkene product. Therefore, the first alkene product is more stable. The base in this reaction is OH, which is not bulky, so the first and second products are the major products.

From the stability of the product formed, the major E1 product is drawn.
(e)
Interpretation:
For the given substrate, when
Concept introduction:
As the number of alkyl groups on the carbon bonded to the leaving group increases, the rate of E1 reaction increases. Each additional alkyl group, which is electron donating, stabilizes the carbocation intermediate produced and thus helps the leaving group leave. The
Answer to Problem 9.69P
The major E1 product for the given reaction is shown below:
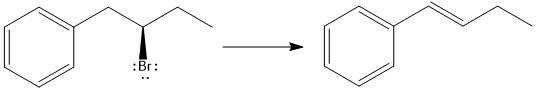
Explanation of Solution
Elimination usually takes place so as to produce the most stable that means highly alkyl substituted alkene.
The possible E1 products are obtained by eliminating the leaving group and a proton on the carbon adjacent to the one bonded to the leaving group. Here the
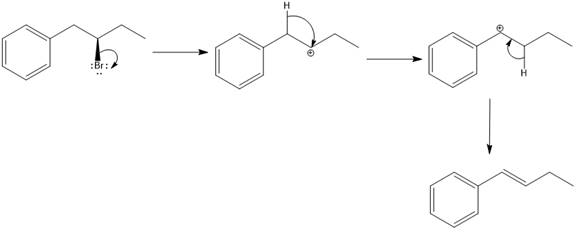
From the stability of the product formed, the major E1 product is drawn.
Want to see more full solutions like this?
Chapter 9 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- When cyclopentene is treated with Br2 in the presence of CH2Cl2 solvent, the major substrate product is __ .arrow_forwardDraw all possible E2 products.arrow_forwardFor each pair of compounds, say which compound is the best SN2 substrate (d) 1-chloro-2,2-dimethylbutane or 2-chlorobutane (e) 1-iodobutane or 2-iodopropanearrow_forward
- draw products, indicate major product, and indicate whether Sn1 Sn2 E1 or E2arrow_forwardBetween E1 and E2, which reaction mechanism is most efficient to synthesize the (E) stereoisomer of this product on an industrial scale?arrow_forwardThis molecule (see image) undergoes a substitution reaction through an SN1 pathway when warmed and stirred with HBr. Draw the two substitution products of this reaction. Show the correct stereochemistry by using wedges and dashes at all chiral centers.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
