
Concept explainers
(a)
Interpretation:
The carbon-carbon triple bond energy is to be calculated. Also, the calculated value and the theoretical value are to be compared.
Concept introduction:
The heat of the reaction
The formula to calculate
Or,
The bond energy of reactants is positive and the bond energy of products is negative.
(a)
Answer to Problem 9.73P
The carbon-carbon triple bond energy is
Explanation of Solution
The given chemical equation for the combustion of ethyne is as follows:
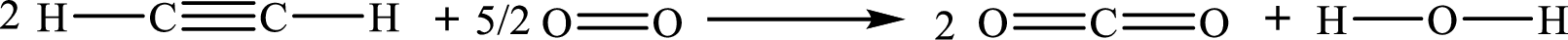
The formula to the enthalpy of the given reaction is as follows:
Rearrange the equation (1) to calculate
Substitute
The value is close to the theoretical value
The carbon-carbon triple bond energy is
(b)
Interpretation:
The heat released when acetylene burn is to be determined.
Concept introduction:
The expression to calculate the moles of solute when given mass and molecular mass of compound are given is as follows:
Stoichiometry of a reaction is utilized to determine the amount of any species in the reaction by the relationship between the reactants and products.
Consider the general reaction,
One mole of
(b)
Answer to Problem 9.73P
The heat released when acetylene burn is
Explanation of Solution
The formula to calculate the moles of acetylene is as follows:
Substitute
The heat released by one mole is
The heat released when acetylene burn is
(c)
Interpretation:
The mass of
Concept introduction:
The expression to calculate the moles of solute when given mass and molecular mass of compound are given is as follows:
Stoichiometry of a reaction is utilized to determine the amount of any species in the reaction by the relationship between the reactants and products.
Consider the general reaction,
One mole of
(c)
Answer to Problem 9.73P
The mass of
Explanation of Solution
The formula to calculate the mass of
Substitute
The mass of
(d)
Interpretation:
The volume of
Concept introduction:
The ideal gas equation can be expressed as follows,
Here,
(d)
Answer to Problem 9.73P
The volume of
Explanation of Solution
The formula to calculate the moles of
Substitute
Rearrange the equation (5) to calculate the volume of
Substitute the value
The volume of
Want to see more full solutions like this?
Chapter 9 Solutions
Chemistry: The Molecular Nature of Matter and Change
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





