
Concept explainers
(a)
Interpretation:
Concept introduction:
The heat of the reaction
The formula to calculate
Or,
The bond energy of reactants is positive and the bond energy of products is negative.
The standard enthalpy of reaction is calculated by the summation of standard enthalpy of formation of the product minus the summation of standard enthalpy of formation of product at the standard conditions. The formula to calculate the standard enthalpy of reaction
Here, m and n are the stoichiometric coefficients of reactants and product in the balanced chemical equation.
(a)
Answer to Problem 9.91P
Explanation of Solution
The given chemical equation for the gas-phase hydration of ethylene to ethanol is as follows:
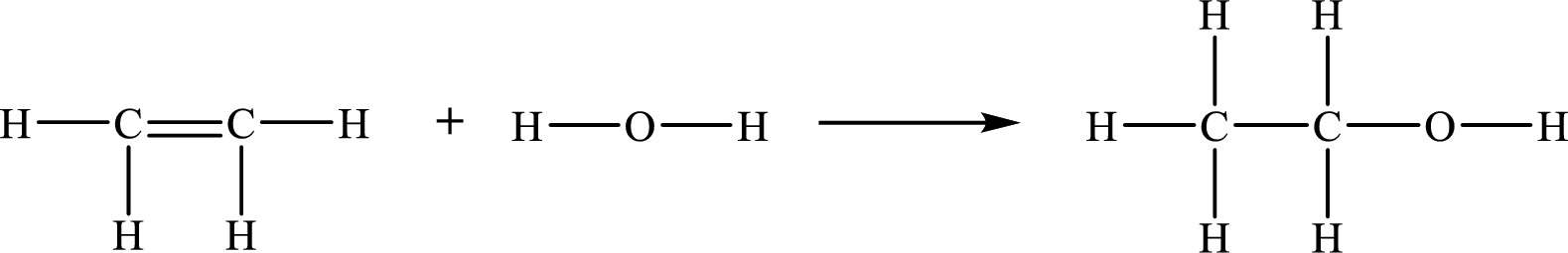
The number of broken bonds is
The number of bonds formed is
The formula to the enthalpy of the given reaction is as follows:
Substitute
The formula to calculate the standard enthalpy of reaction
Substitute
(b)
Interpretation:
Concept introduction:
The heat of the reaction
The formula to calculate
Or,
The bond energy of reactants is positive and the bond energy of products is negative.
(b)
Answer to Problem 9.91P
Explanation of Solution
The given chemical equation for the hydrolysis of ethylene glycol is as follows:
The number of broken bonds is
The number of bonds formed is
The formula to the enthalpy of the given reaction is as follows:
Substitute
(c)
Interpretation:
The reason for the relatively closer value for the hydration in part (a) but not that close for the hydrolysis in part (b) is to be determined.
Concept introduction:
Bond energy is the amount of energy needed to break a bond between two gaseous atoms. Bond energy is measured in
The formula to calculate
Or,
The bond energy of reactants is positive and the bond energy of products is negative.
(c)
Answer to Problem 9.91P
In part (b), the same number of similar bonds are broken in the reactant side and formed in the product side so the value of the enthalpy change is zero. Bond energy is generally specific for a bond and remains the same in different molecules.
Explanation of Solution
Bond energy is generally specific for a bond and depends upon the strength of the bond. The bond energy value is the average value so remain same in different molecules.
In part (b), the same number of similar bonds are broken in the reactant side and formed in the product side so the value of the enthalpy change is zero.
For example, the value of bond energy of
The bond energy value is the average value so the bond energy of the same bond in different molecules remains same.
Want to see more full solutions like this?
Chapter 9 Solutions
Chemistry: The Molecular Nature of Matter and Change
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





