
Concept explainers
(a)
Interpretation:
Concept introduction:
The heat of the reaction
The formula to calculate
Or,
The bond energy of reactants is positive and the bond energy of products is negative.
(a)
Answer to Problem 9.90P
Explanation of Solution
The given chemical equation for the formation of dimethyl ether
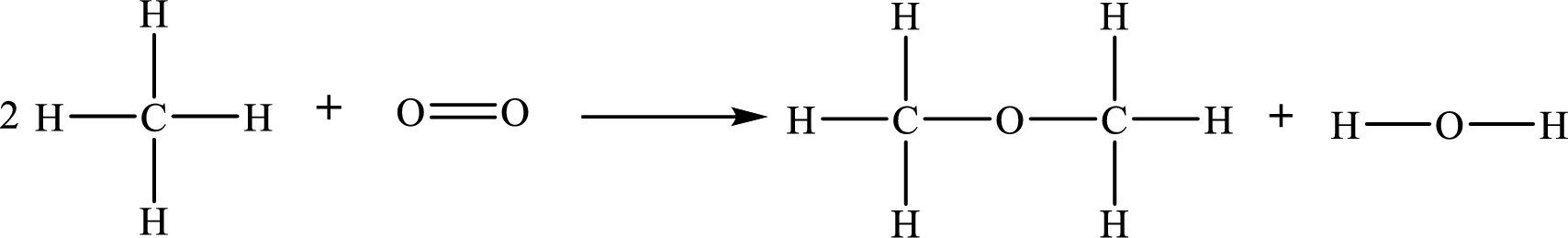
The number of broken bonds is
The number of bonds formed is
The formula to the enthalpy of the given reaction is as follows:
Substitute
The given chemical equation for the formation of ethanol
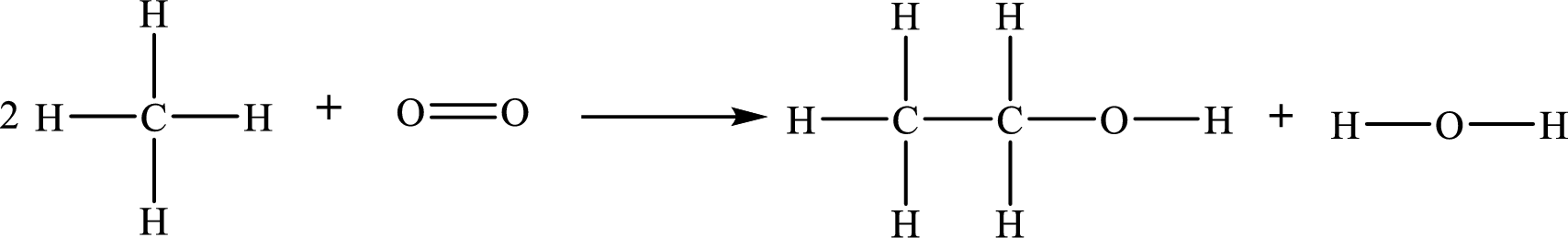
The number of broken bonds is
The number of bonds formed is
The formula to the enthalpy of the given reaction is as follows:
Substitute
(b)
Interpretation:
Among the
Concept introduction:
In the case of a reaction, the change in enthalpy
Here,
Endothermic reactions are the reactions in which energy in the form of the heat or light is absorbed by the reactant for the formation of the product.
Exothermic reactions are the reactions in which energy in the form of the heat or light is released with the product.
(b)
Answer to Problem 9.90P
The formation reaction of ethanol is more exothermic as compared to dimethyl ether.
Explanation of Solution
The value of
Exothermic reactions are the reactions in which energy in the form of the heat or light is released with the product.
(c)
Interpretation:
Concept introduction:
Hess’s law is used to calculate the enthalpy change of an overall reaction that can be derived as a sum of two or more reaction. According to Hess’s law
Enthalpy is a state function so the value depends upon the initial state and final state not on the path so
(c)
Answer to Problem 9.90P
Explanation of Solution
The enthalpy change of the following reaction is
The enthalpy change of the following reaction is
Reverse the equation (2).
The enthalpy change for the reaction (3) is calculated as,
Add equation (1) and (3).
The enthalpy change of the final reaction (4) is
The expression to calculate
Substitute
Want to see more full solutions like this?
Chapter 9 Solutions
Chemistry: The Molecular Nature of Matter and Change
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





