
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
6th Edition
ISBN: 9781305687875
Author: Gilbert
Publisher: CENGAGE LEARNING - CONSIGNMENT
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 9.2, Problem 15E
Interpretation Introduction
Interpretation: The relative reactivity of the H atoms in 1-chlorobutane needs to be determined depending on the percentage calculation of dichlorobutane formed in the reaction.
Concept introduction: 1-Chlorobutane has a chemical formula as
Following are the isomeric products formed when a 1-chlorobutane is monochlorinated using sulfuryl chloride with ABCN as the catalytic agent:
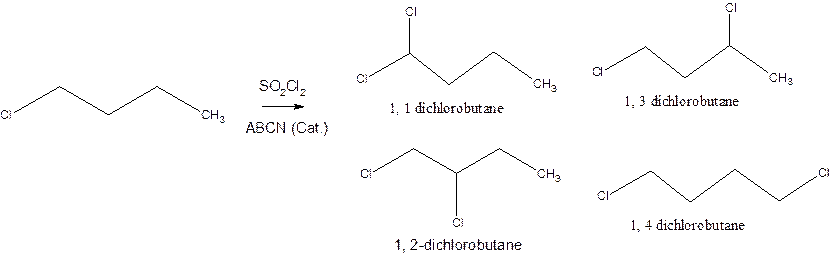
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
p-dihydroxybenzene and o-dihydroxybenzene can be separated by TLC using a solvent of medium polarity. The ortho isomer has a significantly higher Rf value than the para isomer. Suggest a reason for this difference in Rf values. (hint- consider the structure of these two molecules)
Explain 'Conrotatory' and 'Disrotatory' processes in relation to opening of cyclobutane ring.
Give and observation and inference when the compound hexane is subjected into test for Br2 in CCl4
Chapter 9 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
Ch. 9.2 - Prob. 1ECh. 9.2 - Prob. 2ECh. 9.2 - Prob. 3ECh. 9.2 - Prob. 4ECh. 9.2 - Prob. 5ECh. 9.2 - Prob. 6ECh. 9.2 - Prob. 7ECh. 9.2 - Prob. 8ECh. 9.2 - Prob. 9ECh. 9.2 - Prob. 10E
Ch. 9.2 - Prob. 11ECh. 9.2 - Prob. 12ECh. 9.2 - Prob. 13ECh. 9.2 - Prob. 14ECh. 9.2 - Prob. 15ECh. 9.2 - Prob. 16ECh. 9.2 - Prob. 17ECh. 9.2 - Prob. 18ECh. 9.2 - Prob. 19ECh. 9.2 - Prob. 20ECh. 9.2 - Prob. 21ECh. 9.2 - Prob. 22ECh. 9.2 - Prob. 23ECh. 9.2 - Prob. 24ECh. 9.3 - Prob. 1ECh. 9.3 - Prob. 2ECh. 9.3 - Prob. 3ECh. 9.3 - Prob. 4ECh. 9.3 - Prob. 5ECh. 9.3 - Prob. 6ECh. 9.3 - Prob. 7ECh. 9.3 - Prob. 8ECh. 9.3 - Prob. 9ECh. 9.3 - Prob. 10ECh. 9.3 - Prob. 11ECh. 9.3 - Prob. 12ECh. 9.3 - Prob. 13ECh. 9.3 - Prob. 14E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is difference between stereoselective and stereospecific reaction? justify your answer with suitable example.arrow_forwardBromine is known to abstract a secondary hydrogen 97 times as rapidly as a primary hydrogen. Predict the product ratio (or percent yield of each product) for the monobromination of n-butane.arrow_forwardHow do you rationalize the fact that the cyclohexane A value for phenyl (2.8) is bigger than that for isopropyl (2.21)?arrow_forward
- What would be the product ratio in the chlorination of propane if all the hydrogens were abstracted at equal rates?arrow_forwardHow many kinds of chemically non-equivalent hydrogens are there in each of the following compounds?arrow_forwardWrite the reaction of aldehyde with HCN and the energy diagram of the reaction anf interpet product distribution.arrow_forward
- please explain and discuss thoroughly the method of isolating betacarotenearrow_forward1. Write down all the steps of the reaction of 2-Methyl-2-hexene with sulfuric acid and name the product formed. Will provide helpful ratings for correct solution.arrow_forwardI am studying homolytic and heterolytic cleavage. In the halogenation of methane, a methyl radical is formed as an intermediate which indicates homolytic cleavage. Why is the cleavage homolytic and not heterolytic? What is the main determinate of when heterolytic or homolytic cleavage occurs? How big does the electronegativty difference need to be?arrow_forward
- Answer the following question for 1,3,5- hexatriene, the conjugated triene containing six carbons. Within the HOMO, is the phase at the terminal carbons the same or different?arrow_forwardPlease write down the resulting products. How can I know which of the two substituents already present is more strongly conducting in the case of double-substituted benzene? Is generally ortho preferred to para? In the example of OH and NH2 both otho,para conducting. So are there three possible products?arrow_forwardExplain how the peaks in this spectrum occur. Especially the one at 77, which involves two fragmentations. The structure this corresponds to is 1-phenyl-1-butanonearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT