
Concept explainers
(a)
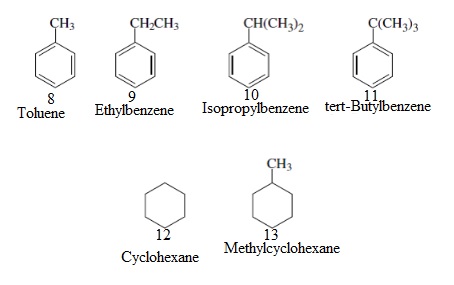
Interpretation: Statistically the most likely compound to form a mono-bromide, for substrates 8-13, needs to be explained.
Concept Introduction: A bromide that contains only one bromine atom in the molecule is called a mono-bromide.
(b)
Interpretation: The most likely compound to form a single isomer of a monobromide for substrates 8-13, needs to be explained.
Concept Introduction: Compounds or radicals having the same type of atoms in the same numbers, and varying in properties and structures, is called a single isomer.
(c)
Interpretation: The compound that is expected to produce the most stable radical, for substrates 8-13, needs to be explained.
Concept Introduction: The neighboring elements which are rich in electrons, stabilize radicals. Thus the least stable radical will be the primary radical and the most stable radical will be tertiary radical.
(d)
Interpretation: The most compound that is expected to produce the least stable radical for substrates 8-13, needs to be explained.
Concept Introduction: Primary radicals are the least stable radicals.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
- Chemistry does methyl bromide favour SN1 or SN2 reactions or can be either depending on conditions ?arrow_forwarda) Write down the products that will occur when you extract HBr from 2-bromo-3-methyl butane in a basic medium, State the reaction conditions. Show which product is the main product. b) Does the main product show the geometric isomer, so please write together. If it shows write the isomers. c) Write the product that will be formed when the main product reacts with KMnO4 in a basic environment in coldarrow_forwardHighlight all of the sites where the halogen could be added upon reaction with HBr.arrow_forward
- In an SN1 reaction, Nucleophile- What are the characteristics of a good nucleophile for SN1? How does a chemist tell a strong versus weak or bulky versus not bulky nucleophile? Solvent-What is the best type of solvent for this reaction? Define the terms please.arrow_forwardwhat would the product be for this reaction 2,3, or 4? and why?arrow_forwarda) Write down the products that will occur when you withdraw HBr from 2-bromine-3-methyl butane in a basic medium, State the reaction conditions. Show which product is the main product. b) Does the main product show the geometric isomer, so please write together. If it shows write the isomers. c) Write the product that will be formed when the main product reacts with KMnO4 in a basic environment in cold.arrow_forward
- for (4) and (5) please provide reactants and why it was chosearrow_forwardExplain why free-radical halogenation usually gives mixtures of productsarrow_forwardThe methoxy group is reported as having σpara and σmeta values that differ in sign (σmeta =+0.12; σpara = –0.27). What specific experiments are performed to determine these s values?Explain why the σpara and σmeta values are of different sign and what those signs indicate.arrow_forward
- Chemistry Please provide detailed mechanisms for this reaction by using the RuCl3 catalyst. What are the roles of HIO4, CCl4, MeCN, H2O? Thanks so much!arrow_forwardExplain why 9-fluorenone has a yellowish color while fluorenol does not. Please be detailed in your explanationarrow_forwardQuestion: How do quantum mechanical effects influence the stability and reactivity of molecules with non-classical carbocations, such as the 2-norbornyl cation, and how does this impact the reaction mechanisms and outcomes?arrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


