
Concept explainers
(a)
Interpretation: The characteristics of a molecule to act as a good initiator needs to be explained.
Concept Introduction: The initiator is used in the initiation step and it is used to start a
(b)
Interpretation: The reason for the unnecessary use of stoichiometric amount of the initiator needs to be explained.
Concept Introduction: Only a catalytic amount of initiator is required for initiation of reaction, further to which there is no role for the initiator in the chemical reaction.
(c)
Interpretation: For the given radical initiators, the bond homolysis needs to be shown using the curved arrow when they are heated or irradiated.
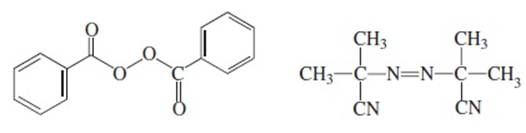
Benzoyl peroxide 2,2'-Azobis
Concept Introduction: In the bond dissociation, the movement of electrons always takes place from negative to positive charge. Thus, the direction of the curved arrow is also from negative to the positive charge.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
- The following excerpt is a commonly used lab procedure for a Diels-Alder reaction. Provide three specific reasons why the one here would not be classified as a green chemistry option compared to ours. Be sure to reference and explain the principles of green chemistry to support your response. excerpt- Place 2 g of maleic anhydride in a 50-mL Erlenmeyer flask and dissolve it in 8 mL of ethyl acetate by warming on a hot plate. Add 8 mL of hexane or petroleum ether, and then cool the solution in an ice bath. Obtain 2 mL freshly cracked cyclopentadiene and add it to the maleic anhydride solution. Swirl to mix. Wait until the product crystallizes from solution, then heat it on the hot plate to redissolve, and allow it to recrystallize. Collect the product by suction filtration, and record the melting point, weight, and percentage yield.arrow_forwardChemistry does methyl bromide favour SN1 or SN2 reactions or can be either depending on conditions ?arrow_forwardFriedel-Crafts Alkylation, based on substituted benzene reaction, including reactivity based on electron donating and withdrawing group and also discuss the possible positions (Ortho, Meta and Para) for the new group. Explain if there is polysubstitution occurs. Keeping the reaction mixture in the ice bath and adding the reagent slowly by continuously stirring is the key point of success.arrow_forward
- The Diels–Alder Reaction – A [4+2] Cycloaddition Used: - Maleic Anhydride - Furan Summarize the Rf values for the starting material and product. Explain any differences or similarities in the Rf values based on molecular structure. Are the Rf values useful in analyzing the reaction?arrow_forwardbased on the results, what is the characteristic of sn1 and sn2 reactions generallyarrow_forwardWhat are the final products for these reactions? *mechanisms not necessaryarrow_forward
- can i get help drawing out actual structures including the nucleophilic addition of Cy2NH to parafomaldehyde and its hemiaminal intermidiate and the condensation step when it is displaced by terminal alkyne forming allene, also what is dioxane getting rid of as the solvent, thanksarrow_forwardWhat is the delocalization energy and π-bond formation energy of (i) the allyl radical, (ii) the cyclobutadiene cation?arrow_forward1) Show the steps for the free radical chlorination of toluene using Cl2 and light to give benzyl chloride. 2) Demonstrate resonance forms for the benzyl radical that is the principal intermediate, showing how the benzene ring stabilizes this radical.arrow_forward
- Show a detailed arrow pushing reaction for the Diels-Alder reaction between a-phellandrene and maleic anhydride. Be sure to show the Endo and exo isomers and show how each would be formed.arrow_forwardComment on the reactivity of 2-chlorobutane and 2-bromobutane in both Sn1 and Sn2 reaction. Is your data according to the theoretical prediction?arrow_forwardWhy is tertiary carbocation most favored for Sn1 reactions?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


