
a)
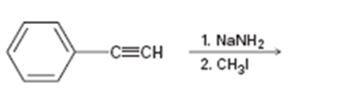
Interpretation:
The product of the two step process shown is to be given and complete electron-pushing mechanism for its formation also is to be provided.
Concept introduction:
The terminal
To propose:
The product of the two step process shown and to give a complete electron-pushing mechanism for its formation.
b)
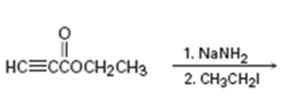
Interpretation:
The product of the two step process shown is to be given and complete electron-pushing mechanism for its formation also is to be provided.
Concept introduction:
The terminal alkynes being acidic form sodium alkynides when treated with bases like sodium amide. The alkynides when treated with alkyl halides yield the higher alkynes as the product. The alkynide ion being nucleophilic attacks the positively polarized carbon of C-X bond in alkyl halides displaces the halogen to yield the higher alkyne as the product.
To propose:
The product of the two step process shown and to give a complete electron-pushing mechanism for its formation.
c)
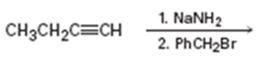
Interpretation:
The product of the two step process shown is to be given and complete electron-pushing mechanism for its formation also is to be provided.
Concept introduction:
The terminal alkynes being acidic form sodium alkynide when treated with bases like sodium amide. The alkynides when treated with alkyl halides yield the higher alkynes as the product. The alkynide ion being nucleophilic attacks the positively polarized carbon of C-X bond in alkyl halides displaces the halogen to yield the higher alkyne as the product.
To propose:
The product of the two step process shown and to give a complete electron-pushing mechanism for its formation.
Trending nowThis is a popular solution!

Chapter 9 Solutions
ORGANIC CHEMISTRY-EBOOK>I<
- single reaction sequence: a certain ketone undergoes alkylation to give new ketone, when reacted with a base and then an alkylating agent, 1-bromopropane. What is the structure of the final ketone product?arrow_forward47) Provide the structure of the major organic product which results in the following reaction. Br KI Br CH3arrow_forward4) Draw the complete electron-pushing arrow mechanism for the following reductions. Explain, using resonance contributors, the regiochemistry that results in each case. ỌMe Na, MeOH ? NH3 CHO Na, MeOH ? NH3arrow_forward
- 7) Provide a synthesis of the following compounds using the given starting material and any other reagents. so,H starting material final product он он starting material final productarrow_forwardA problem often encountered in the oxidation of primary alcohols to acids is that esters are sometimes produced as by-products. For example, oxidation of ethanol yields acetic acid and ethyl acetate: Propose a mechanism to account for the formation of ethyl acetate. Take into account the reversible reaction between aldehydes and alcohols:arrow_forwardNonconjugated , -unsaturated ketones, such as 3-cyclohexenone, are in an acid-catalyzed equilibrium with their conjugated , -unsaturated isomers. Propose a mechanism for this isomerization.arrow_forward
- 1) Draw the complete electron-pushing arrow mechanism for the following reductions. Explain, using resonance contributors (structures), the regiochemistry that results in each case. OMe Na, MeOH ? NH3 CHO Na, MeOH ? NH3arrow_forward3) Draw the structures of the major organic product(s) for the following reactions. HI in CH₂Cl₂ 3 excess Cl₂ in H₂O H₂SO4 in H₂O (Two products) Br-Cl in THF 1) BH3 2) alkaline H₂O₂ OSO4 + HOOHarrow_forwardb) The Wolf-Kishner reduction is a reaction used in Organic Chemistry to convert carbonyl functionalities into methylene group. The reaction was used to convert an aldehyde or ketone to an alkane using hydrazine, base and thermal conditions. The mechanism begins with the attack of hydrazine of the aldehyde or ketone. Stage 1: The reaction of aldehyde/ketone with hydrazine to produce hydrazine Stage 2: Reaction with the base and heat to convert hydrozone to alkane Write the mechanism of the reaction.arrow_forward
- Complete synthesis, show reagents and intermediate compounds.arrow_forwardComplete the following multistep syntheses by showing the major products and the reagents nessesary for each steparrow_forward(a) Propose a mechanism for the conversion of cis-hex-3-ene to the epoxide (3,4-epoxyhexane)and the ring-opening reaction to give the glycol, hexane-3,4-diol. In your mechanism, payparticular attention to the stereochemistry of the intermediates and products.(b) Repeat part (a) for trans-hex-3-ene. Compare the products obtained from cis- andtrans-hex-3-ene. Is this reaction sequence stereospecific?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

