
Concept explainers
(a)
Interpretation: The structures of
Concept introduction: Organic compound that consists of only carbon and hydrogen atoms in them are called hydrocarbons. These can either be saturated or unsaturated. Saturated hydrocarbons are compounds that contain only single bonds between them while hydrocarbons with multiple bonds are called
(b)
Interpretation: The structures of
Concept introduction: Organic compound that consists of only carbon and hydrogen atoms in them are called hydrocarbons. These can either be saturated or unsaturated. Saturated hydrocarbons are compounds that contain only single bonds between them while hydrocarbons with multiple bonds are called unsaturated hydrocarbons. Alkanes are saturated hydrocarbons with general formula of
(c)
Interpretation: The structure of
Concept introduction: Organic compound that consists of only carbon and hydrogen atoms in them are called hydrocarbons. These can either be saturated or unsaturated. Saturated hydrocarbons are compounds that contain only single bonds between them while hydrocarbons with multiple bonds are called unsaturated hydrocarbons. Alkanes are saturated hydrocarbons with general formula of
(d)
Interpretation: The structure of
Concept introduction: Organic compound that consists of only carbon and hydrogen atoms in them are called hydrocarbons. These can either be saturated or unsaturated. Saturated hydrocarbons are compounds that contain only single bonds between them while hydrocarbons with multiple bonds are called unsaturated hydrocarbons. Alkanes are saturated hydrocarbons with general formula of
(e)
Interpretation: The structure of
Concept introduction: Organic compound that consists of only carbon and hydrogen atoms in them are called hydrocarbons. These can either be saturated or unsaturated. Saturated hydrocarbons are compounds that contain only single bonds between them while hydrocarbons with multiple bonds are called unsaturated hydrocarbons. Alkanes are saturated hydrocarbons with general formula of
(f)
Interpretation: The structure of
Concept introduction: Organic compound that consists of only carbon and hydrogen atoms in them are called hydrocarbons. These can either be saturated or unsaturated. Saturated hydrocarbons are compounds that contain only single bonds between them while hydrocarbons with multiple bonds are called unsaturated hydrocarbons. Alkanes are saturated hydrocarbons with general formula of
(g)
Interpretation: The structure of
Concept introduction: Organic compound that consists of only carbon and hydrogen atoms in them are called hydrocarbons. These can either be saturated or unsaturated. Saturated hydrocarbons are compounds that contain only single bonds between them while hydrocarbons with multiple bonds are called unsaturated hydrocarbons. Alkanes are saturated hydrocarbons with general formula of
(h)
Interpretation: The structure of
Concept introduction: As per IUPAC recommendations longest chain found in a continuous manner in a branched molecule is chosen as parent chain. Any substituent that protrudes from certain carbon of parent chain is termed side chain. In hydrocarbon with branches the chief side chains are illustrated below:
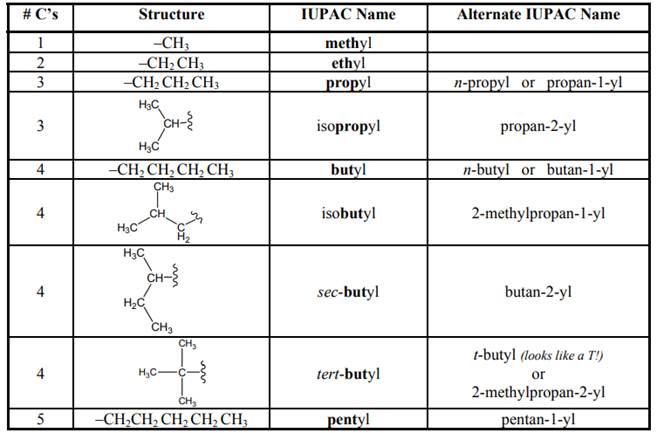
The numeral present before “yl” indicate the carbon number of side chain that is used to bind as the side chain to parent chain.
(i)
Interpretation: The structure of
Concept introduction: Organic compound that consists of only carbon and hydrogen atoms in them are called hydrocarbons. These can either be saturated or unsaturated. Saturated hydrocarbons are compounds that contain only single bonds between them while hydrocarbons with multiple bonds are called unsaturated hydrocarbons. Alkanes are saturated hydrocarbons with general formula of
(i)
Interpretation: The structure of
Concept introduction: The convention followed for IUPAC nomenclature of cyclic alkanes is as follows:
- If even a single ring is found in a molecule, this ring is regarded as the parent chain.
- Alkyl groups attached to rings are assigned smallest priority and number and it is followed in cyclic manner toward the closest group.
- If two alkyl; group happen to be same the smallest priority number is assigned alphabetically.
Trending nowThis is a popular solution!

Chapter NW1 Solutions
Organic Chemistry: A Guided Inquiry
- A student names the second structure above 2,3-dimethylcyclohex-1-ene. What rule does thisviolate?arrow_forwardBreak down the following hydrocarbons names plz. I also have to draw the compound plzarrow_forwardMark the reactions in Figure 21 that will produce an alcohol as the main organic product. * A B Carrow_forward
- ALL ONE QUESTION THANKS Make a line and 3d model of each molecule below A.) What type of bonds are in each molecule B.) which bonds allow rotationarrow_forwardWhat does the UV light do to the molecules? What information is determined from the UV spectra?arrow_forwardd) when you react ammonia with a halogenated alkane will you get only one organic product? Why or why not? (do not talk about inorganic products)arrow_forward
- What will be the color of the flame and the amount of soot if the following are ignited:(a) Hexane (b)Heptane(c) Cyclohexane (d) Cyclohexene (e) Benzene (f) Toluenearrow_forwardOrganic Chemistry 1 Question. EA24. Please show why the answer is B. letter , and not D. letter . Thank youarrow_forward(E) What suffix do all the names in Model 1 have in common with each other?arrow_forward
- a. C4H8 + Cl2 -----> C4H8Cl2 b. C6H6 + Cl2 -----> C6H5Cl + HCl c. C3H6 + HCl -----> C3H7Cl Do they have an addition reaction? please explainarrow_forwardfor part b)why do we write AlCl3 at top of the arrow and AlCl4 at the bottom of arrow??is it any connection between the two?does it means that AlCl 4 need to be present in order to make AlCl3 reacts to the molecule and make the reaction to process?? 2. why when AlCl3 added,Cl was removed from the ring?what princeiple theory is that ? 2. when SO3 is added ,SO3 attached to the ring,why ?what principle is behind this phenamonane 3.Just confused about why AlCl3 removes Cl from the ring,while HSO3 donate SO3 to the ring. Is it Something about nucleohilic or eletrciohillic?arrow_forwardWrite a detail note on Micheal Addition reaction 1,2 vs 1,4 addition reactions?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


