1A H 2A 3A 4A SA 6A 7A He LI Be BCNOF Ne Na Mg 38 4B 5B 6B 7B 88 1B 28 A1 Si PS CI Ar KCa Sc TiV Cr Mn Fe Co Ni Cu 2n Ga Ge As Se Br Kr Rb Sr Y ZrND Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La HfTa W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa UNp Pu Am Cm Bk Cf Es Fm Md NoLr Arrange the following ions in order of increasing ionic radius: magnesium ion, nitride ion, fluoride ion, aluminum ion Enter the FORMULA for each ion in the boxes below. Smallest Largest
1A H 2A 3A 4A SA 6A 7A He LI Be BCNOF Ne Na Mg 38 4B 5B 6B 7B 88 1B 28 A1 Si PS CI Ar KCa Sc TiV Cr Mn Fe Co Ni Cu 2n Ga Ge As Se Br Kr Rb Sr Y ZrND Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La HfTa W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa UNp Pu Am Cm Bk Cf Es Fm Md NoLr Arrange the following ions in order of increasing ionic radius: magnesium ion, nitride ion, fluoride ion, aluminum ion Enter the FORMULA for each ion in the boxes below. Smallest Largest
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and e xplain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
100%
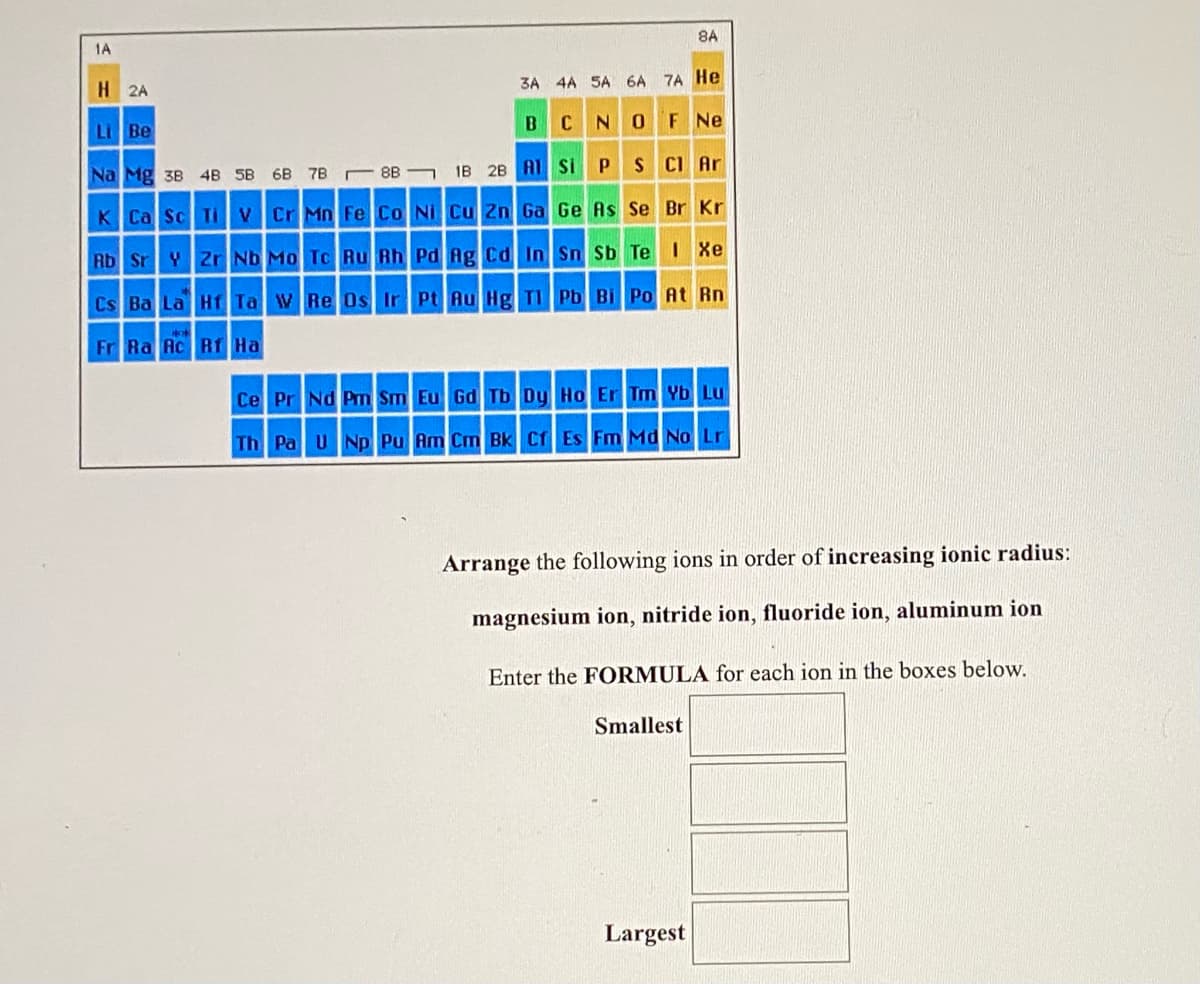
Transcribed Image Text:8A
1A
H 2A
3A 4A SA 6A 7A He
B C
NOF Ne
Li Be
E 8B
1B 28 Al Si PS CI Ar
Na Mg 3B 4B 5B 6B 7B
K Ca Sc Ti
VCr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
Rb SrY 2r Nb Mo Tc Ru Rh Pd Ag cd In S Sb Te IXe
Cs Ba La Hf Ta WRe 0s Ir Pt Au Hg TI Pb Bi Po At Rn
Fr Ra Ac Rf Ha
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
Th Pa UNp Pu Am Cm Bk Cf Es Fm Md No Lr
Arrange the following ions in order of increasing ionic radius:
magnesium ion, nitride ion, fluoride ion, aluminum ion
Enter the FORMULA for each ion in the boxes below.
Smallest
Largest
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
