7.22°C I NH,NO, 300mL Time 600ML 500ML 400ML 300mL 200ML 100mL 200mL 100mL SOLIDS SOLUTIONS | Cation H20 SOLUTIONS LIQUIDS Anion DSוJ SOLIDS Water - H20 RUN EXPERIMENT amonium nitrate - NH4NO3 Show graph view Mass (g) 100. Mass (g) 20.0 Show microscopic view Temp (°C) 20.0 Temp (°C) 20.0 Replay Reset Show AH solution (kJ/mol) 25.69 X Show specific heat (J/g°C) 4.184 Temperature
7.22°C I NH,NO, 300mL Time 600ML 500ML 400ML 300mL 200ML 100mL 200mL 100mL SOLIDS SOLUTIONS | Cation H20 SOLUTIONS LIQUIDS Anion DSוJ SOLIDS Water - H20 RUN EXPERIMENT amonium nitrate - NH4NO3 Show graph view Mass (g) 100. Mass (g) 20.0 Show microscopic view Temp (°C) 20.0 Temp (°C) 20.0 Replay Reset Show AH solution (kJ/mol) 25.69 X Show specific heat (J/g°C) 4.184 Temperature
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter15: Energy And Chemical Change
Section: Chapter Questions
Problem 77A
Related questions
Question
How much energy, in kilojoules, did the water lose in the picture? (Use q = (m)(c)(ΔT) and then convert joules to kilojoules.)
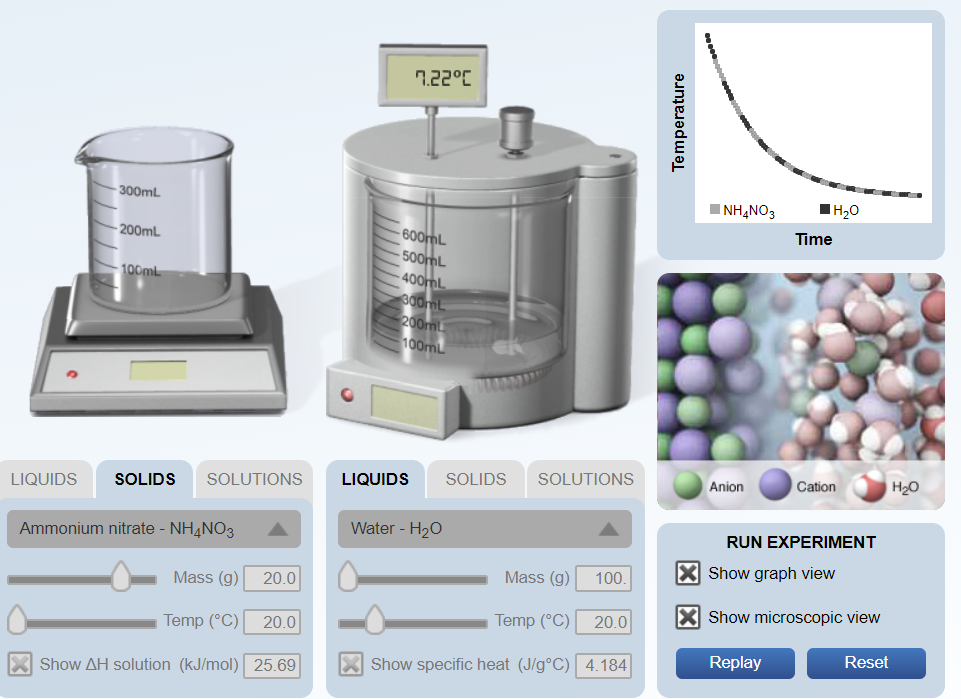
Transcribed Image Text:7.22°C
300mL
INH,NO3
IH20
Time
-600ML
500ML
400ML
300mL
200mL
100mL
200mL
100mL
SOLIDS
SOLUTIONS
Cation
H20
LIQUIDS
Anion
LIQUIDS
SOLIDS
SOLUTIONS
Water - H20
RUN EXPERIMENT
Ammonium nitrate - NH,NO3
X Show graph view
Mass (g)
100.
Mass (g)
20.0
X Show microscopic view
Temp (°C)
20.0
Temp (°C)
20.0
Reset
Replay
X Show specific heat (J/g°C) 4.184
X Show AH solution (kJ/mol) 25.69
Temperature
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
