A sample of pure water was spiked with 0.520 ng/mL silver ion. Ten replicate determinations of the spiked water sample gave 0.530, 0.510, 0.487, 0.473, 0.514, 0.494, 0.498, 0.502, 0.480, and 0.465 ng/mL silver ion. Determine the mean percent recovery of the spike and the detection limit of the analytical method used for the silver ion determination. mean percent recovery: % detection limit: ng/mL
A sample of pure water was spiked with 0.520 ng/mL silver ion. Ten replicate determinations of the spiked water sample gave 0.530, 0.510, 0.487, 0.473, 0.514, 0.494, 0.498, 0.502, 0.480, and 0.465 ng/mL silver ion. Determine the mean percent recovery of the spike and the detection limit of the analytical method used for the silver ion determination. mean percent recovery: % detection limit: ng/mL
Chapter16: Data Processing With Excel
Section: Chapter Questions
Problem 5P
Related questions
Question
100%
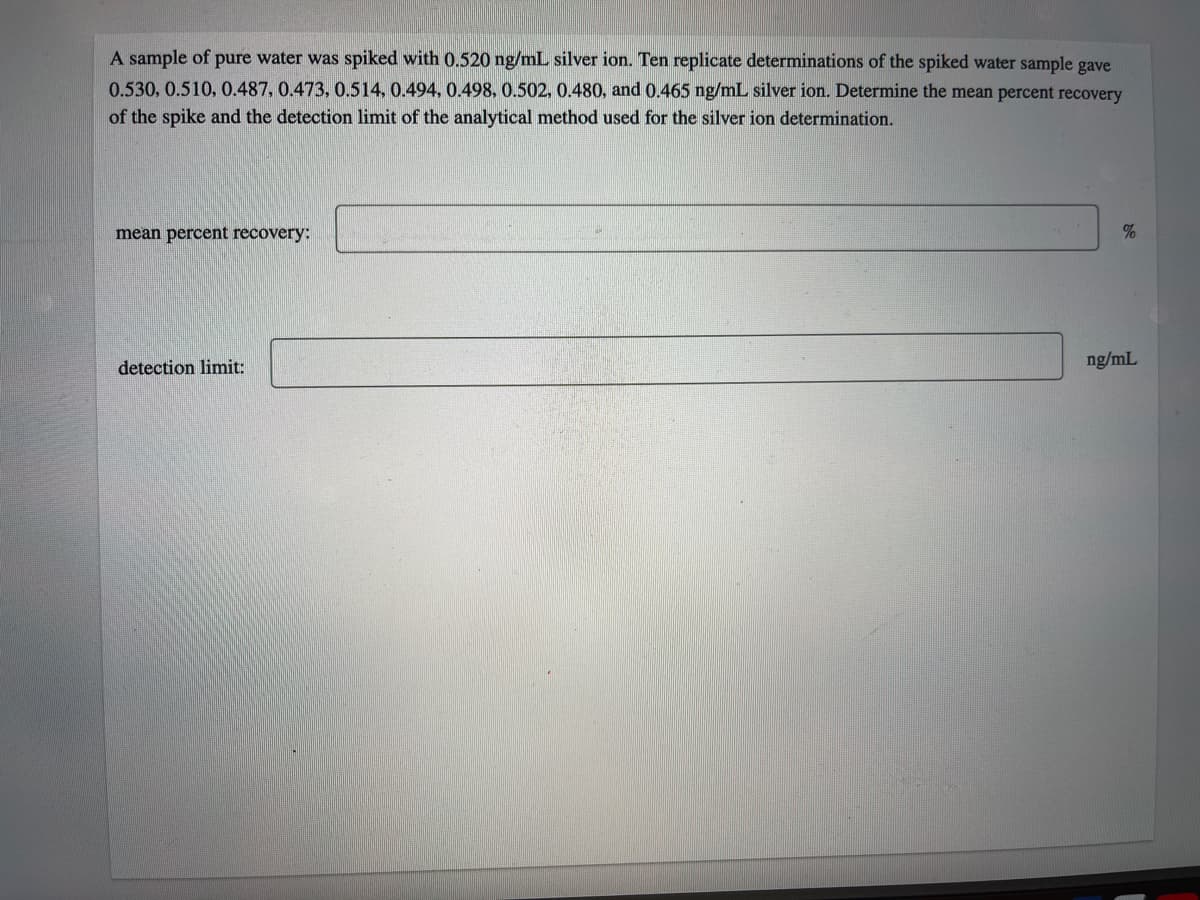
Transcribed Image Text:A sample of pure water was spiked with 0.520 ng/mL silver ion. Ten replicate determinations of the spiked water sample gave
0.530, 0.510, 0.487, 0.473, 0.514, 0.494, 0.498, 0.502. 0.480, and 0.465 ng/mL silver ion. Determine the mean percent recovery
of the spike and the detection limit of the analytical method used for the silver ion determination.
mean percent recovery:
detection limit:
ng/mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
