b) The thermogravimetric curve for calcium salicylate monohydrate (CAC14H1006-H2O) is shown below. (mw 332 g/mol). Starting with an initial sample mass of 16.55 mg, calculate the mass of the sample remaining when it is heated to i) 350 oc and ii) 800 oc. The first step is loss Of H20 (mw 18 g/mol), followed by loss Of C703H6 (mw 138 g/mol), then C6H4 (mw 76 g/mol) and finally C02 (mw 44 g/mol). Give your answers to two decimal places and show your work. CaC,,H06 H,O CaC,,H,0 20- 40 CaC,0,H, 60 CaCO, CaO 80 100 200 800 Temperature ("C) 400 600 1000 Mass loss (%)
b) The thermogravimetric curve for calcium salicylate monohydrate (CAC14H1006-H2O) is shown below. (mw 332 g/mol). Starting with an initial sample mass of 16.55 mg, calculate the mass of the sample remaining when it is heated to i) 350 oc and ii) 800 oc. The first step is loss Of H20 (mw 18 g/mol), followed by loss Of C703H6 (mw 138 g/mol), then C6H4 (mw 76 g/mol) and finally C02 (mw 44 g/mol). Give your answers to two decimal places and show your work. CaC,,H06 H,O CaC,,H,0 20- 40 CaC,0,H, 60 CaCO, CaO 80 100 200 800 Temperature ("C) 400 600 1000 Mass loss (%)
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.19QAP
Related questions
Question
100%
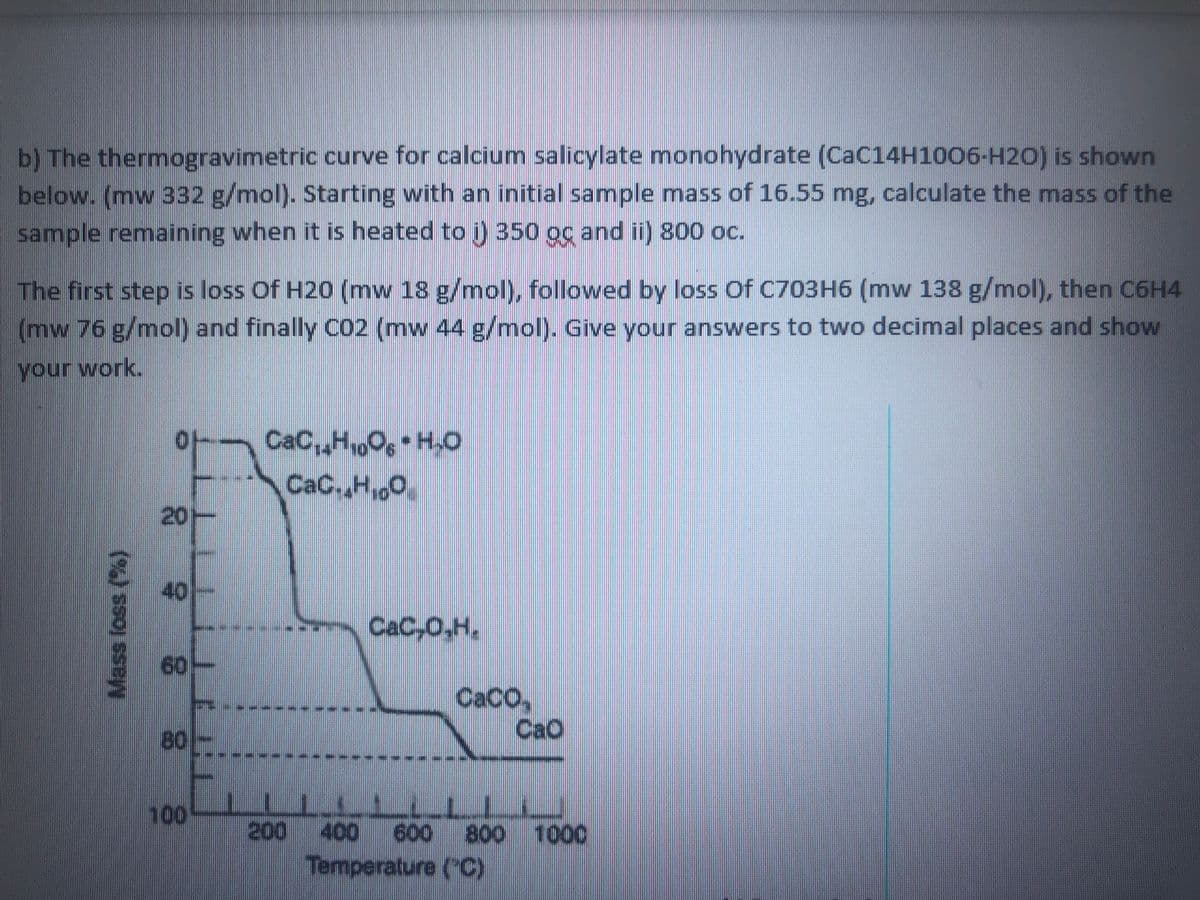
Transcribed Image Text:b) The thermogravimetric curve for calcium salicylate monohydrate (CaC14H1006-H20) is shown
below. (mw 332 g/mol). Starting with an initial sample mass of 16.55 mg, calculate the mass of the
sample remaining when it is heated to ) 350 oc and ii) 800 oc.
The first step is loss Of H20 (mw 18 g/mol), followed by loss Of C703H6 (mw 138 g/mol), then C6H4
(mw 76 g/mol) and finally CO2 (mw 44 g/mol). Give your answers to two decimal places and show
your work.
CaC,,HO, H.O
CaC.,H,0
of
20-
40
CaC,0.H,
60-
CACO,
CaO
80
100
200 400 600 800 100
Temperature (C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning