Calculate the orders of the reaction with respect to I–, BrO3 , and H+ – using the aforementioned systematic approach. In order to simplify this task, the proper experiments are provided. The orders should be rounded to the nearest integer.
Calculate the orders of the reaction with respect to I–, BrO3 , and H+ – using the aforementioned systematic approach. In order to simplify this task, the proper experiments are provided. The orders should be rounded to the nearest integer.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section: Chapter Questions
Problem 36QRT
Related questions
Question
Calculate the orders of the reaction with respect to I–, BrO3 , and H+ –
using the aforementioned systematic approach. In order to simplify this task, the proper experiments are provided. The orders should be rounded to the nearest integer.
![For example, for the [I] for reaction mixture #1, 2 mL of 0.010 M KI was used. So we obtain:
M = (0.010 M· 2 mL)/10 mL
= 0.0020 M
Use this procedure to complete the reactant concentrations for the other four mixtures.
Calculating the Orders of the Reaction
Calculate the orders of the reaction with respect to I, BrO,, and H* using the aforementioned
systematic approach. In order to simplify this task, the proper experiments are provided. The
orders should be rounded to the nearest integer. Include your calculations on the following pages.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fddc1ec45-98e4-4675-b97b-277ce49cbbd0%2F2831b0c0-99ca-4484-a23d-594acfaa1144%2Fpfdz6z9_processed.jpeg&w=3840&q=75)
Transcribed Image Text:For example, for the [I] for reaction mixture #1, 2 mL of 0.010 M KI was used. So we obtain:
M = (0.010 M· 2 mL)/10 mL
= 0.0020 M
Use this procedure to complete the reactant concentrations for the other four mixtures.
Calculating the Orders of the Reaction
Calculate the orders of the reaction with respect to I, BrO,, and H* using the aforementioned
systematic approach. In order to simplify this task, the proper experiments are provided. The
orders should be rounded to the nearest integer. Include your calculations on the following pages.
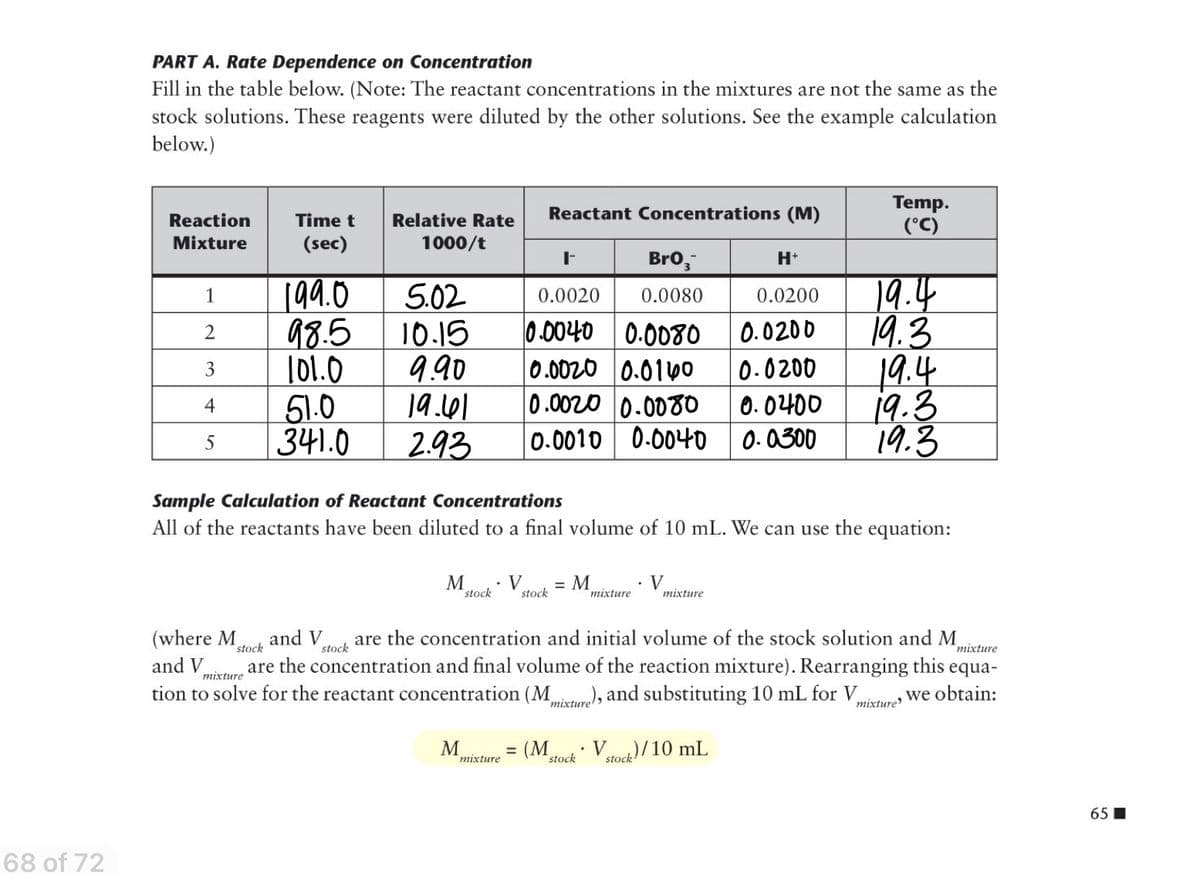
Transcribed Image Text:PART A. Rate Dependence on Concentration
Fill in the table below. (Note: The reactant concentrations in the mixtures are not the same as the
stock solutions. These reagents were diluted by the other solutions. See the example calculation
below.)
Temp.
Reaction
Time t
Relative Rate
Reactant Concentrations (M)
(°C)
Mixture
(sec)
1000/t
Bro,
H*
[9.0
98.5
|01.0
51.0
341.0
5.02
10.15
9.90
ja.101
2.93
19.4
19.3
19.4
19.3
19.3
1
0.0020
0.0080
0.0200
0.0040
0.0020
0.0020
0.0200
0.0080
0.0140
3
0.0200
0.0080
0.0040
0. 0400
0.0300
4
5
0.0010
Sample Calculation of Reactant Concentrations
All of the reactants have been diluted to a final volume of 10 mL. We can use the equation:
M
V
stock
stock
M
V
mixture
mixture
are the concentration and initial volume of the stock solution and M
are the concentration and final volume of the reaction mixture). Rearranging this equa-
tion to solve for the reactant concentration (M ), and substituting 10 mL for Vu Wwe obtain:
(where M
and V
stock
stock
mixture
and V
тіxture
mixture'
mixture
M
mixture
(М.
Vtod) /10 mL
stock
stock
65 I
68 of 72
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,