Continued... 5. Answer ALL parts of this question The stability of chloramphenicol in eye drops is being studied at different temperatures and times. The concentration (in mg/L) of chloramphenicol at three different temperatures over time is provided below. Time (day) -18°C 25°C 40°C 25 28 23 7 27 20 20 30 20 21 20 180 23 25 19 (a) Using the data in the tables below assess and explain if the reaction time (after 1 hour of the start of the reaction) was an important factor affecting the yield of the reaction and provide the null hypothesis of the statistical test carried out. (b) The amount of chloramphenicol at -18 °C seems to vary with some values appearing to be higher than others. Explain what may be causing this variation AND suggest a methodology to identify ||
Continued... 5. Answer ALL parts of this question The stability of chloramphenicol in eye drops is being studied at different temperatures and times. The concentration (in mg/L) of chloramphenicol at three different temperatures over time is provided below. Time (day) -18°C 25°C 40°C 25 28 23 7 27 20 20 30 20 21 20 180 23 25 19 (a) Using the data in the tables below assess and explain if the reaction time (after 1 hour of the start of the reaction) was an important factor affecting the yield of the reaction and provide the null hypothesis of the statistical test carried out. (b) The amount of chloramphenicol at -18 °C seems to vary with some values appearing to be higher than others. Explain what may be causing this variation AND suggest a methodology to identify ||
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter33: Automated Methods Of Analysis
Section: Chapter Questions
Problem 33.8QAP
Related questions
Question
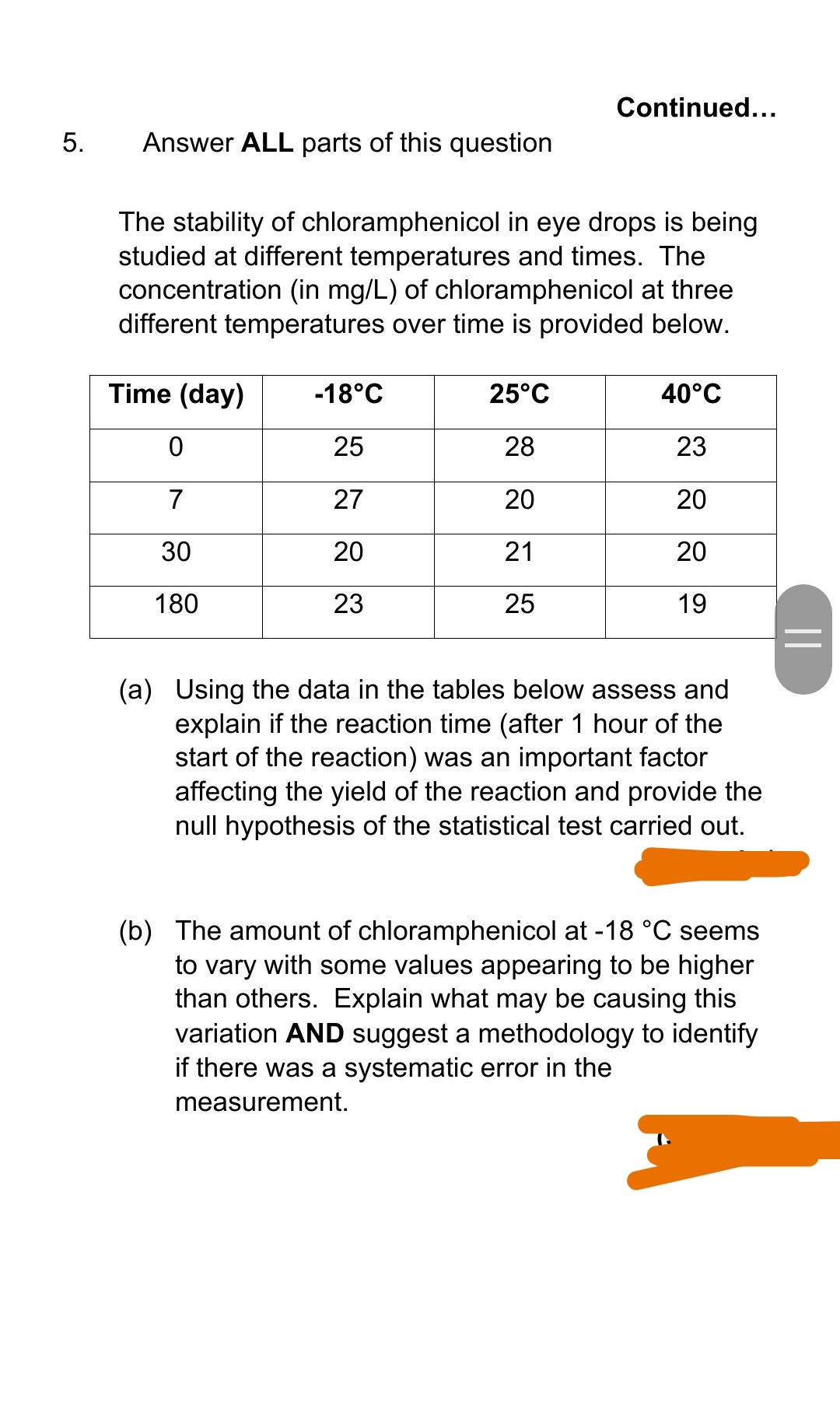
Transcribed Image Text:Continued...
5.
Answer ALL parts of this question
The stability of chloramphenicol in eye drops is being
studied at different temperatures and times. The
concentration (in mg/L) of chloramphenicol at three
different temperatures over time is provided below.
Time (day)
-18°C
25°C
40°C
25
28
23
7
27
20
20
30
20
21
20
180
23
25
19
(a) Using the data in the tables below assess and
explain if the reaction time (after 1 hour of the
start of the reaction) was an important factor
affecting the yield of the reaction and provide the
null hypothesis of the statistical test carried out.
(b) The amount of chloramphenicol at -18 °C seems
to vary with some values appearing to be higher
than others. Explain what may be causing this
variation AND suggest a methodology to identify
if there was a systematic error in the
measurement.
||
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT