The progress of a hypothetical reaction: A + B C+DE, was monitored using paper chromatography. Compounds A and B were mixed and a sample of the solution was spotted every five minutes. Same stationary phase and mobile phase were used throughout the experiment. The figure below shows chromatograms at different time points. Lane R corresponds to the migration of standards A, B, C, D, and E. R O min 5 mins 10 mins 15 mins 20 mins 25 mins 30 mins A. Calculate the retention factor of A, B, C, D, and E given that the order of polarity is D > E> A >C> B. A = B = C D = E = II II
The progress of a hypothetical reaction: A + B C+DE, was monitored using paper chromatography. Compounds A and B were mixed and a sample of the solution was spotted every five minutes. Same stationary phase and mobile phase were used throughout the experiment. The figure below shows chromatograms at different time points. Lane R corresponds to the migration of standards A, B, C, D, and E. R O min 5 mins 10 mins 15 mins 20 mins 25 mins 30 mins A. Calculate the retention factor of A, B, C, D, and E given that the order of polarity is D > E> A >C> B. A = B = C D = E = II II
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section11.6: Properties Of Liquids
Problem 1.2ACP
Related questions
Question
I need help on this problem. And please only round off on the final answer.
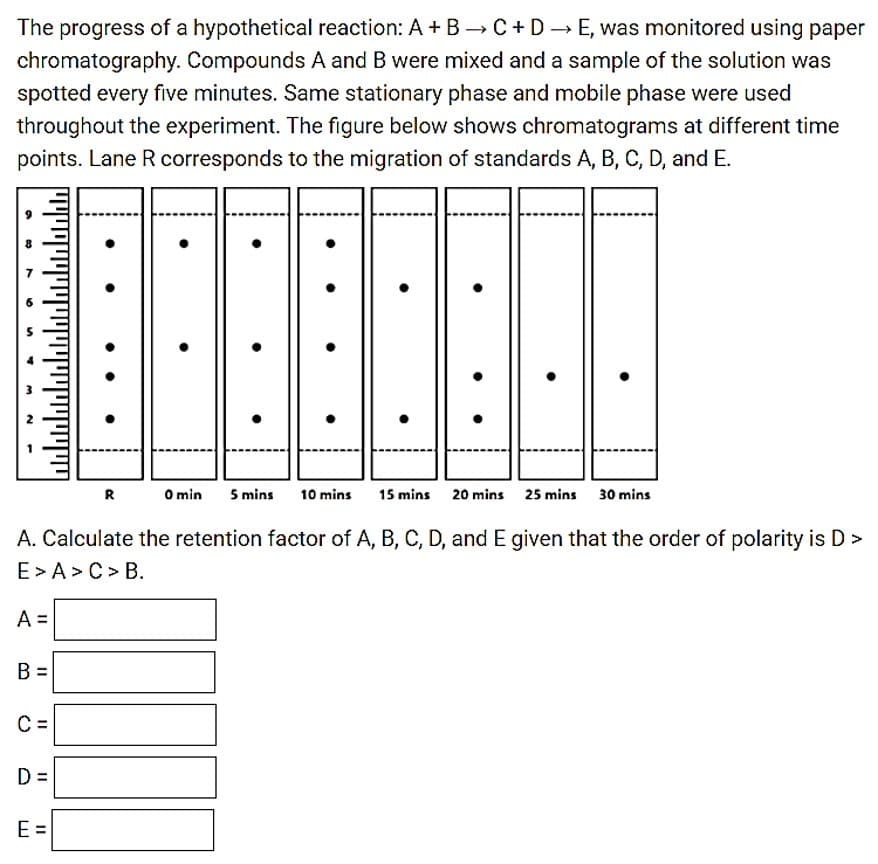
Transcribed Image Text:The progress of a hypothetical reaction: A + B →C+D E, was monitored using paper
chromatography. Compounds A and B were mixed and a sample of the solution was
spotted every five minutes. Same stationary phase and mobile phase were used
throughout the experiment. The figure below shows chromatograms at different time
points. Lane R corresponds to the migration of standards A, B, C, D, and E.
R
O min
5 mins
10 mins
15 mins
20 mins 25 mins 30 mins
A. Calculate the retention factor of A, B, C, D, and E given that the order of polarity is D >
E> A>C> B.
A =
B =
C
D =
E =
II II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole
