When 2-methylcyclohexanone is treated with pyrrolidine, two isomeric enamines are formed. H+ + H,O H. A (85%) В (15%) Why is enamine A with the less substituted double bond the thermodynamically favored product? (You will find it helpful to examine the models of these two enamines.)
When 2-methylcyclohexanone is treated with pyrrolidine, two isomeric enamines are formed. H+ + H,O H. A (85%) В (15%) Why is enamine A with the less substituted double bond the thermodynamically favored product? (You will find it helpful to examine the models of these two enamines.)
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter23: Amines
Section: Chapter Questions
Problem 23.72P
Related questions
Question
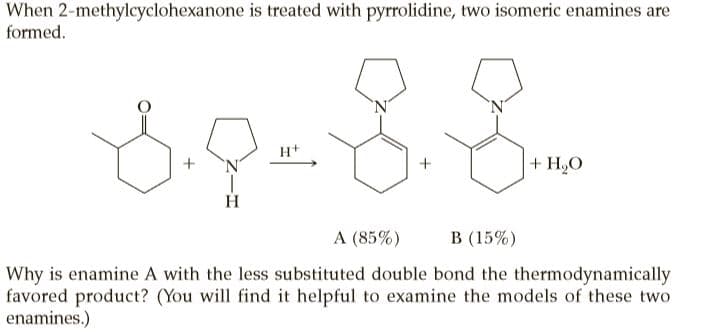
Transcribed Image Text:When 2-methylcyclohexanone is treated with pyrrolidine, two isomeric enamines are
formed.
H+
+ H,O
H.
A (85%)
В (15%)
Why is enamine A with the less substituted double bond the thermodynamically
favored product? (You will find it helpful to examine the models of these two
enamines.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning