
Concept explainers
(a)
Interpretation:
For the given line structure, a Lewis structure is to be drawn including all the lone pairs.
Concept introduction:
Line structures are compact like condensed structures. When drawing line structures, carbon atoms and the hydrogen atoms attached to them are not drawn explicitly. A carbon atom is implied at the intersection of two bonds and at the end of each bond. Atoms other than carbon and hydrogen are shown. Non-bonding electrons are usually not shown unless they are important to emphasize an aspect of the atom.
Answer to Problem 1.63P
For the given line structure, the structure with all lone pairs and hydrogen atoms is:
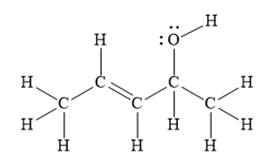
Explanation of Solution
The given line structure is:
In the given line structure, there is a five carbon chain, with a carbon-carbon double bond and a hydroxyl group. The hydrogen atoms are attached to each carbon atom such that each carbon atom forms four bonds in all. The hydroxyl group is attached to one of the carbon atoms of the chain. There should be two lone pairs of electrons on the oxygen atom so as to complete its octet. Thus, the structure with all carbon atoms, hydrogen atoms, and lone pairs is as shown below:
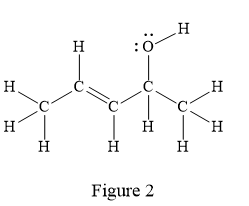
The Lewis structure for the given line structure including lone pairs and hydrogen atoms is shown in Figure 2 above.
(b)
Interpretation:
For the given line structure, a Lewis structure is to be drawn including all the lone pairs.
Concept introduction:
Line structures are compact like condensed structures. When drawing line structures, carbon atoms and the hydrogen atoms attached to them are not drawn explicitly. A carbon atom is implied at the intersection of two bonds and at the end of each bond. Atoms other than carbon and hydrogen are drawn. Non-bonding electrons are usually not shown unless they are important to emphasize an aspect of the atom.
Answer to Problem 1.63P
For the given line structure, the structure with all lone pairs and hydrogen atoms is:
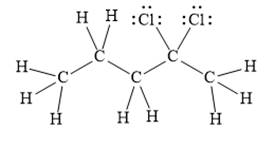
Explanation of Solution
The given line structure is:
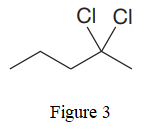
In the given line structure, there is a five carbon chain with two chlorine atoms attached to one of the carbon atoms in the chain. A carbon atom is implied at the intersection of two bonds and at the end of each bond. The hydrogen atoms are attached to each carbon atom such that each carbon atom forms four bonds in all. Two chlorine atoms are attached to one of the carbon atoms of the chain. There must be three lone pairs of electrons on each chlorine atom so that its octet is complete. Thus, the structure with all carbon atoms, hydrogen atoms, and lone pairs is as shown below:
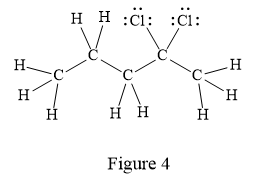
The Lewis structure for the given line structure including lone pairs and hydrogen atoms is shown in Figure 4 above.
(c)
Interpretation:
For the given line structure, a Lewis structure is to be drawn including all the lone pairs.
Concept introduction:
Line structures are compact like condensed structures. When drawing line structures, carbon atoms and the hydrogen atoms attached to them are not drawn explicitly. A carbon atom is implied at the intersection of two bonds and at the end of each bond. Atoms other than carbon and hydrogen are drawn. Non-bonding electrons are usually not shown unless they are important to emphasize an aspect of the atom.
Answer to Problem 1.63P
For the given line structure, the structure with all lone pairs and hydrogen atoms is:
Explanation of Solution
The given line structure is:
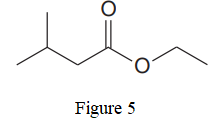
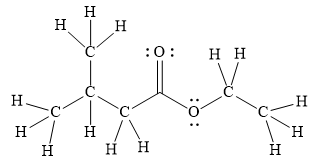
In the given line structure, there is a chain of four carbon atoms on the left side of a singly bonded oxygen. An ethyl fragment is present at the right side of the singly bonded oxygen atom. The structure has two oxygen atoms, a doubly bonded and a singly bonded, so, each of the oxygen must carry two lone pairs so that their octet is complete. A carbon atom is implied at the intersection of two bonds and at the end of each bond. The hydrogen atoms are attached to each carbon atom such that each carbon atom forms four bonds in all.
Thus, the structure with all carbon atoms, hydrogen atoms, and lone pairs is as shown below:
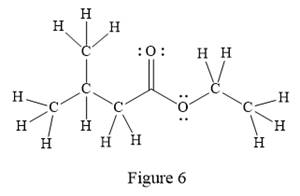
The Lewis structure for the given line structure including lone pairs and hydrogen atoms is shown in Figure 6 above.
(d)
Interpretation:
For the given line structure, a Lewis structure is to be drawn including all the lone pairs.
Concept introduction:
Line structures are compact like condensed structures. When drawing line structures, carbon atoms and the hydrogen atoms attached to them are not drawn explicitly. A carbon atom is implied at the intersection of two bonds and at the end of each bond. Atoms other than carbon and hydrogen are drawn. Non-bonding electrons are usually not shown unless they are important to emphasize an aspect of the atom.
Answer to Problem 1.63P
For the given line structure, the structure with all lone pairs and hydrogen atoms is:
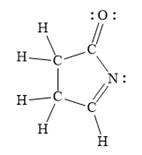
Explanation of Solution
The given line structure is:
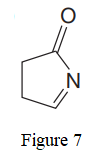
In the given line structure, a five membered ring containing a nitrogen atom is present. One of the carbon atoms in the ring forms a double bond with the oxygen atom. A carbon atom is implied at the intersection of two bonds and at the end of each bond. The hydrogen atoms are attached to each carbon atom such that each carbon atom forms four bonds in all. Nitrogen atom should have a lone pair of electrons so as to complete its octet while oxygen atom needs two lone pairs of electrons on it.
Thus, the structure with all carbon atoms, hydrogen atoms, and lone pairs is as shown below:
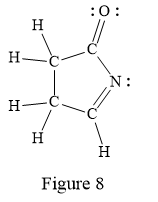
The Lewis structure for the given line structure including lone pairs and hydrogen atoms is shown in Figure 8 above.
(e)
Interpretation:
For the given line structure, a Lewis structure is to be drawn including all the lone pairs.
Concept introduction:
Line structures are compact like condensed structures. When drawing line structures, carbon atoms and the hydrogen atoms attached to them are not drawn explicitly. A carbon atom is implied at the intersection of two bonds and at the end of each bond. Atoms other than carbon and hydrogen are drawn. Non-bonding electrons are usually not shown unless they are important to emphasize an aspect of the atom.
Answer to Problem 1.63P
For the given line structure, the structure with all lone pairs and hydrogen atoms is:
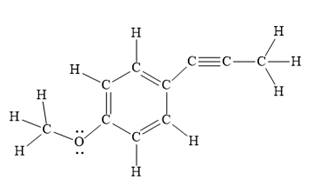
Explanation of Solution
The given line structure is:
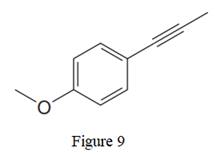
In the above line structure, the disubstituted benzene ring is present. One substituent of the benzene ring is a three carbon chain with an internal triple bond. The other substituent is a methoxy group,
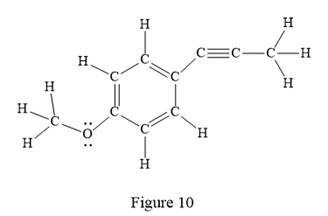
The Lewis structure for the given line structure including lone pairs and hydrogen atoms is shown in Figure 10 above.
(f)
Interpretation:
For the given line structure, a Lewis structure is to be drawn including all the lone pairs.
Concept introduction:
Line structures are compact like condensed structures. When drawing line structures, carbon atoms and the hydrogen atoms attached to them are not drawn explicitly. A carbon atom is implied at the intersection of two bonds and at the end of each bond. Atoms other than carbon and hydrogen are drawn. Non-bonding electrons are usually not shown unless they are important to emphasize an aspect of the atom.
Answer to Problem 1.63P
For the given line structure, the structure with all lone pairs and hydrogen atoms is:
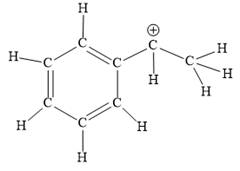
Explanation of Solution
The given line structure is:
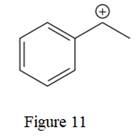
The above line structure is a structure for a cation. A carbocation is a carbon bearing a positive formal charge which is explicitly shown in the Lewis structure. A six membered carbon ring with alternate double and single bonds is present. A carbon atom is implied at the intersection of two bonds and at the end of each bond. The hydrogen atoms are attached to each carbon atom such that each carbon atom forms four bonds in all. The carbon bearing a positive charge must possess three bonds.
Thus, the structure with all carbon atoms, hydrogen atoms, and lone pairs is as shown below:
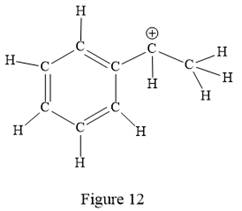
The Lewis structure for the given line structure including lone pairs and hydrogen atoms is shown in Figure 12 above.
(g)
Interpretation:
For the given line structure, a Lewis structure is to be drawn including all the lone pairs.
Concept introduction:
Line structures are compact like condensed structures. When drawing line structures, carbon atoms and the hydrogen atoms attached to them are not drawn explicitly. A carbon atom is implied at the intersection of two bonds and at the end of each bond. Atoms other than carbon and hydrogen are drawn. Non-bonding electrons are usually not shown unless they are important to emphasize an aspect of the atom.
Answer to Problem 1.63P
For the given line structure, the structure with all lone pairs and hydrogen atoms is:
Explanation of Solution
The given line structure is:
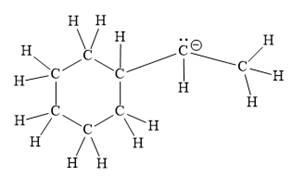
The above line structure is a structure for an anion. A six membered carbon ring with single bonds is present. The negatively charged carbon atom must possess three bonds and one lone pair of electrons. A carbon atom is implied at the intersection of two bonds and at the end of each bond. The hydrogen atoms are attached to each carbon atom such that each carbon atom forms four bonds in all.
Thus, the structure with all carbon atoms, hydrogen atoms, and lone pairs is as shown below:
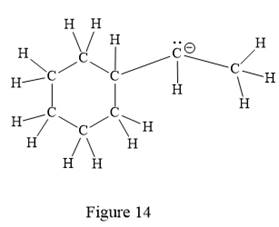
The Lewis structure for the given line structure including lone pairs and hydrogen atoms is shown in Figure 14 above.
(h)
Interpretation:
For the given line structure, a Lewis structure is to be drawn including all the lone pairs.
Concept introduction:
Line structures are compact like condensed structures. When drawing line structures, carbon atoms and the hydrogen atoms attached to them are not drawn explicitly. A carbon atom is implied at the intersection of two bonds and at the end of each bond. Atoms other than carbon and hydrogen are drawn. Non-bonding electrons are usually not shown unless they are important to emphasize an aspect of the atom.
Answer to Problem 1.63P
For the given line structure, the structure with all lone pairs and hydrogen atoms is:
Explanation of Solution
The given line structure is:
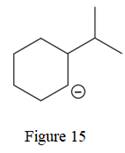
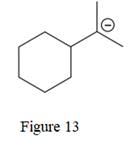
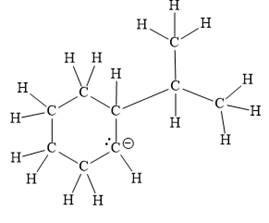
The above line structure is a six membered ring with a negative charge on one of the carbon atoms of the ring. The ring is monosubstituted with an isopropyl group. The negatively charged carbon atom must possess three bonds and one lone pair of electrons. A carbon atom is implied at the intersection of two bonds and at the end of each bond. The hydrogen atoms are attached to each carbon atom such that each carbon atom forms four bonds in all.
Thus, the structure with all carbon atoms, hydrogen atoms, and lone pairs is as shown below:
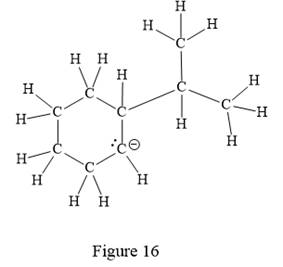
The Lewis structure for the given line structure including lone pairs and hydrogen atoms is shown in Figure 16 above.
(i)
Interpretation:
For the given line structure, a Lewis structure is to be drawn including all the lone pairs.
Concept introduction:
Line structures are compact like condensed structures. When drawing line structures, carbon atoms and the hydrogen atoms attached to them are not drawn explicitly. A carbon atom is implied at the intersection of two bonds and at the end of each bond. Atoms other than carbon and hydrogen are drawn. Non-bonding electrons are usually not shown unless they are important to emphasize an aspect of the atom.
Answer to Problem 1.63P
For the given line structure, the structure with all lone pairs and hydrogen atoms is:
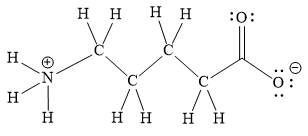
Explanation of Solution
The given line structure is:
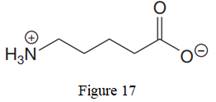
The above line structure is a chain of five carbon atoms. One end of the chain has carboxylate ion, in which the oxygen carries a negative charge. The other end of the chain has a nitrogen atom with three hydrogen atoms directly attached to it and carrying a positive charge. A carbon atom is implied at the intersection of two bonds and at the end of each bond. The hydrogen atoms are attached to each carbon atom such that each carbon atom forms four bonds in all. A singly bonded oxygen with a negative charge must have three lone pairs on it while the doubly bonded oxygen atom should possess two lone pairs.
Thus, the structure with all carbon atoms, hydrogen atoms, and lone pairs is as shown below:
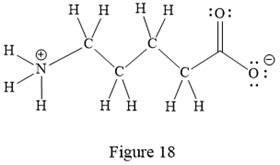
The Lewis structure for the given line structure including lone pairs and hydrogen atoms is shown in Figure 18 above.
Want to see more full solutions like this?
Chapter 1 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Provide all the possible resonance structure for the following speciesarrow_forwardDo not give handwriting solution. What is the formal charge of structure C?arrow_forwardwhy is ethane a nonpolar covalent bond? I get that it is because they have "equal" shared valence electrons- but how do we know that? It seems they are on opposite sides of the periodic table, so I thought they have vastly different electronegativity.arrow_forward
- Draw the structure of the pentanedioate anion. Be sure to include the charge(s).arrow_forwardThe formal charge on the phosphorus atom in the resonance structures for the phosphate ion that minimizes the formal changes is ?arrow_forwardGive a clear handwritten answer with explanation....give all possible resonance structures with different pi bond positions. Give also lone pairs in the structuresarrow_forward
- Provide ALL reasonable resonance structures for each molecular structure below. Note that you need use the curved arrows to show how electrons are moved and please make sure your answers are legit Lewis structure (with lone pairs).arrow_forwardThree resonance structures are possible for dinitrogen monoxide, N2O. (a) Draw the three resonance structures. (b) Calculate the formal charge on each atom in each resonance structure. (c) Based on formal charges and electronegativity, predict which resonance structure is the most reasonable.arrow_forwardIt is impossible to draw a legitimate Lewis structure of a neutral NH4 molecule. Hypothetically,how many valence electrons would such a neutral NH4 molecule have ifit could exist? a. The +1 cation, NH4+ , does exist. How many valence electrons does one NH4+ ion have? b. Draw the Lewis structure for NH4+arrow_forward
- Draw the contributing structure indicated by the curved arrow(s). Show all valence electrons and all formal chargesarrow_forwardDraw all the possible resonance structures for the nitrite ion. How many resonance structures can you have in total for nitrite?arrow_forwardWhat do you mean by the below statement ? An inductive effect is the pull of electron density through σ bondscaused by electronegativity differences of atoms.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning




