
Concept explainers
(a)
Interpretation: The condensed formula should be converted to hashed and wedged form.
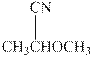
Concept introduction:There are three ways of representation of molecular structures. The hashed and wedged formulas have three types of bonds. The bond above the plane is represented by bold hash while bonds behind the plane are represented by wedge.The bonds in plane are represented by normal bonds.
Bond-line formula depicts the zig-zag framework of carbon chain with attached substituents. The terminal position represents a methyl while the non-terminal is for methylene group. For instance, the propane is written in this notation as follows:
(b)
Interpretation: The condensed formula of
Concept introduction: There are three ways of representation of molecular structures. The hashed and wedged formulas have three types of bonds. The bond above the plane is represented by bold hash while bonds behind the plane are represented by wedge. The bonds in plane are represented by normal bonds.
Bond-line formula depicts the zig-zag framework of carbon chain with attached substituents. The terminal position represents a methyl while the non-terminal is for methylene group. For instance the propane is written in this notation as follows:
(c)
Interpretation: The condensed formula of
Concept introduction: There are three ways of representation of molecular structures. The hashed and wedged formulas have three types of bonds. The bond above the plane is represented by bold hash while bonds behind the plane are represented by wedge. The bonds in plane are represented by normal bonds.
Bond-line formula depicts the zig-zag framework of carbon chain with attached substituents. The terminal position represents a methyl while the non-terminal is for methylene group. For instance the propane is written in this notation as follows:
(d)
Interpretation: The condensed formula should be converted to hashed and wedged form.
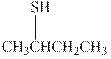
Concept introduction: There are three ways of representation of molecular structures. The hashed and wedged formulas have three types of bonds. The bond above the plane is represented by bold hash while bonds behind the plane are represented by wedge. The bonds in plane are represented by normal bonds.
Bond-line formula depicts the zig-zag framework of carbon chain with attached substituents. The terminal position represents a methyl while the non-terminal is for methylene group. For instance the propane is written in this notation as follows:
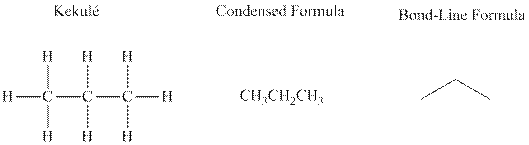
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Organic Chemistry: Structure and Function
- Convert the following condensed structure to a bond-line structure. CHOCH(CH3)CH2CO2CH3arrow_forward2. Which of the following compounds could have configurational isomerism (cis or trans) and if they do, draw the both of the structures and label them as cis and trans d) 1,2-dichloroethene e) 2-methyl-2-butene f) 1-chloropropenearrow_forwardIdentify and draw possible cis and trans isomers of CH3CH2CH(CH3)CHCH(CH3)arrow_forward
- Classify the following hydrocarbons as alkanes, cycloalkane, alkene, cycloalkenes, alkyne, cycloalkyne, or aromatic.a. CH2 CH CH2b. CH3 C (CH3)2 CH (CH2CH3) CH2 CH2 CH (CH3)2c. (CH3)3 C C C CH (CH3) CH2 CH3arrow_forwardwhat is the line structure of (CH3)2CH(CH2)2CHO?arrow_forwardConvert the following into skeletal structures. 1. (CH3)2CHCH2CH(CH3)(CH2)2CH3 2. (CH2CH3)3C(CH2)3CH3arrow_forward
- Draw skeletal structures for the cyclopropane (three-membered ring) isomers with a formula of C₅H₁₀. Note: cyclopropane is a carbon-carbon ring with three carbons. Three isomersarrow_forward. Which of the following compounds exists as cis-trans isomers? Draw the structures of those that do. CH3CH=CHC4H9 (iso) CH3CH2CH=C(CH3) (C2H5)arrow_forwardDraw 2 possible structures for each of the formulas; C6H8 and C6H6arrow_forward
- Chemistry Determine how many substituents in the axial position does the most stable conformer of (1R,2S,4R)-4-bromo-1,2-dimethylcyclohexane have?arrow_forwardWhich of the following CAN exist as cis-trans isomers? I. CH3CH2CH=CHCH3 II. ClCH=CHCl III. (CH3)2C=CHCH3 IV. CH3 (Br)C=C(Br)CH3 CHOICES:I, II, III, IV II, III, IV only I, II, IV only I, II, III onlyarrow_forwardDraw the expanded, condensed and skeletal structure for the compound:a. C4H8b. C3H7OHc. C2H5Brd. CH3COOHarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


