
Concept explainers
Interpretation:
The degeneracies for all energies of a
Concept introduction:
The Schrodinger equation is used to find the allowed energy levels for electronic transitions in the
Where,
•
•
•
The energy obtained after applying the operator on wavefunction is known as the eigen value for the wavefunction.
Answer to Problem 10.81E
The degeneracies for all energies of a
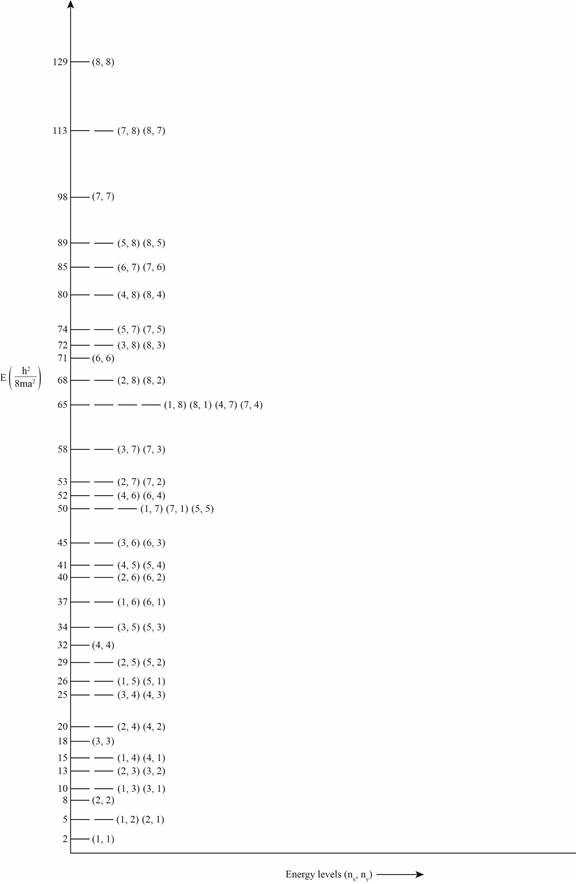
Explanation of Solution
The formula to calculate energy for
Assuming
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energies corresponding to energy levels
Similarly, energy corresponding to energy levels
Similarly, energy corresponding to energy levels
Similarly, energy corresponding to energy levels
Similarly, energy corresponding to energy levels
Similarly, energy corresponding to energy levels
Similarly, energy corresponding to energy levels
The plot of energy versus energy levels is shown in the figure 1.
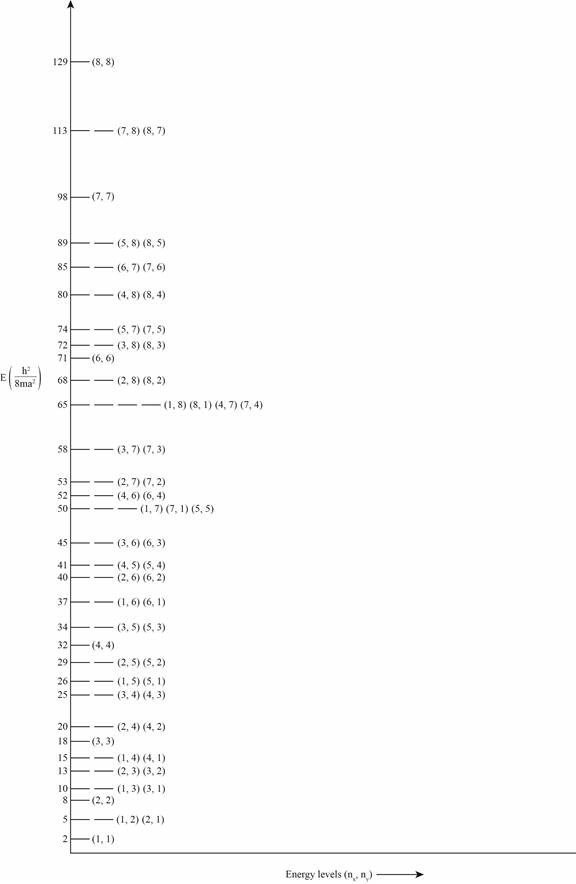
Figure 1
The degeneracies for all energies of a
Want to see more full solutions like this?
Chapter 10 Solutions
Physical Chemistry
- Is the uncertainty principle consistent with our description of the wavefunctions of the 1D particle-in-a-box? Hint: Remember that position is not an eigenvalue operator for the particle-in-a-box wavefunctions.arrow_forwardFor a particle in a state having the wavefunction =2asinxa in the range x=0toa, what is the probability that the particle exists in the following intervals? a x=0to0.02ab x=0.24ato0.26a c x=0.49ato0.51ad x=0.74ato0.76a e x=0.98ato1.00a Plot the probabilities versus x. What does your plot illustrate about the probability?arrow_forwardWhat is the degeneracy of an h subshell? An n subshell?arrow_forward
- A particle on a ring has a wavefunction =12eim where equals 0 to 2 and m is a constant. Evaluate the angular momentum p of the particle if p=i How does the angular momentum depend on the constant m?arrow_forwardShow that the normalization constants for the general form of the wavefunction =sin(nx/a) are the same and do not depend on the quantum number n.arrow_forwardAn official baseball has a mass of 145g. a Assuming that a baseball in New Orleans Superdome width =310m is acting as a particle-in-a-box, what is its energy in the n=1 state? b Assuming that the energy in part a is all kinetic energy (=12mv2), what is the velocity of the baseball in the n=1 state? c A hit baseball can travel as fast as 44.7m/s. Calculate the classical kinetic energy of the hit baseball and, assuming that this energy is quantized, determine the quantum number of the hit baseball.arrow_forward
- Verify that the following wavefunctions are indeed eigenfunctions of the Schrdinger equation, and determine their energy eigenvalues. a =eiKx where V=0 and K is a constant b =eiKx where V=k, k is some constant potential energy, and K is a constant c =2asinxa where V=0.arrow_forwardA particle on a ring has a wavefunction =eim, where =0to2 and m is a constant. a Normalize the wavefunction, where d is d. How does the normalization constant depend on the constant m? b What is the probability that the particle is in the ring indicated by the angular range =0to2/3? Does this answer make sense? How does the probability depend on constant m?arrow_forwardIn exercise 10.41a, the wavefunction is not normalized. Normalize the wavefunction and verify that it still satisfies the Schrdinger equation. The limits on x are 0 and 2. How does the expression for the energy eigenvalue differ?arrow_forward
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

