
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 10, Problem 4Q
Use the single-
TABLE 10.1 Single-bond homolytic dissociation energies DH° at 25°C
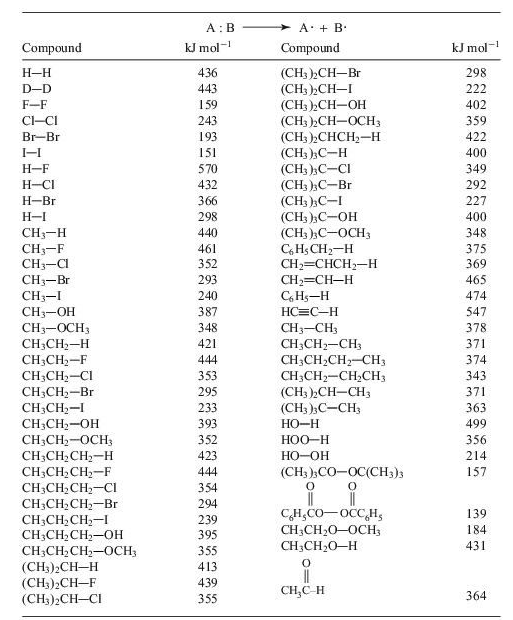
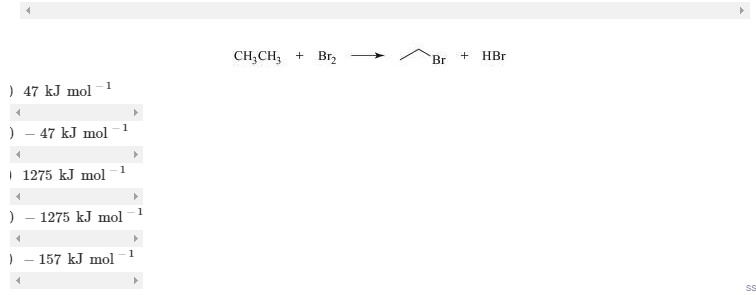
10.4 Using the data of Table 10.1, calculate the heat of reaction, ΔH°, of the reaction,
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A.) What is the heat of reaction, ΔH°?
CO2(g) + H2O(l) à H2CO3(aq)
–20.2 kJ mol–1
–1379 kJ mol–1
–592 kJ mol–1
B.)
What is the average bond energy in CO2?
CO2(g) ΔH°f, = –393.5 kJ mol–1
CO(g) ΔH°f, = –110.5 kJ mol–1
C(g) ΔH°f, = +715 kJ mol–1
CO32–(aq) ΔH°f, = –676.3 kJ mol–1
O(g) ΔH°f, = +249.0 kJ mol–1
207 kJ mol–1
1607 kJ mol–1
804 kJ mol–1
19
Hydrogenation of double and triple bonds is an important industrial process. Calculate (in kJ) the standard enthalpy change ΔH° for the hydrogenation of ethyne (acetylene) to ethane using average bond enthalpies (use exam data sheet values).
H–C≡C–H(g) + 2H2(g) → H3C–CH3(g)
Given that ∆H° for the reaction is -42 kcal/mol and the bond dissociation enthalpies for the C - H, C - Cl, and O - H bonds are 101, 85, and 105 kcal/mol respectively, calculate the bond dissociation enthalpy of the O - Cl bond.
Chapter 10 Solutions
Organic Chemistry
Ch. 10 - Prob. 1PPCh. 10 - Prob. 2PPCh. 10 - Practice Problem 10.3 How would the molecular ion...Ch. 10 - Prob. 4PPCh. 10 - Prob. 5PPCh. 10 - Prob. 6PPCh. 10 - Practice Problem 10.7 Chlorination reactions of...Ch. 10 - Prob. 8PPCh. 10 - Prob. 9PPCh. 10 - Prob. 10PP
Ch. 10 - Prob. 11PPCh. 10 - Practice Problem 10.12 Benzylic radicals, due to...Ch. 10 - Prob. 13PPCh. 10 - Practice Problem 10.14 Show how the following...Ch. 10 - Prob. 15PPCh. 10 - Prob. 16PPCh. 10 - Prob. 17PPCh. 10 - Prob. 18PCh. 10 - Explain the relative distribution of produces...Ch. 10 - 10.20 Which of the following compounds can be...Ch. 10 - Prob. 21PCh. 10 - Prob. 22PCh. 10 - Prob. 23PCh. 10 - Prob. 24PCh. 10 - 10.25 List in order of decreasing stability all of...Ch. 10 - Prob. 26PCh. 10 - Prob. 27PCh. 10 - Prob. 28PCh. 10 - Starting with the compound or compounds indicated...Ch. 10 - Prob. 30PCh. 10 - 10.31 Synthesize each of the following compounds...Ch. 10 - 10.32 Synthesize each of the following compounds...Ch. 10 - Prob. 33PCh. 10 - Prob. 34PCh. 10 - Prob. 35PCh. 10 - Write a mechanism for the following reaction.Ch. 10 - Prob. 37PCh. 10 - The halogen atom of an alkyl halide can be...Ch. 10 - Prob. 39PCh. 10 - Prob. 40PCh. 10 - If one were to try to draw the simplest Lewis...Ch. 10 - Prob. 1LGPCh. 10 - 2. (a) Propose a synthesis of 2-methoxypropene...Ch. 10 - Use the single-bond dissociation energies of Table...Ch. 10 - 10.2 In the radical chlorination of methane, one...Ch. 10 - Prob. 3QCh. 10 - Use the single-bond dissociation energies of Table...Ch. 10 - Prob. 5QCh. 10 - Prob. 6Q
Additional Science Textbook Solutions
Find more solutions based on key concepts
Draw the structures of straight-chain alcohols that have from one to six carbons with an OH group at the end of...
Essential Organic Chemistry (3rd Edition)
Practice Exercise 2
Calculate the pH of a solution containing 0.085 M nitrous acid (HNO2, Ka = 4.5 x 10-4) an...
Chemistry: The Central Science (14th Edition)
Refrigerant- 134a is being transported a 0.1 kg/s through a Teflon tube of inside diameter D0=28 mm and outside...
Fundamentals of Heat and Mass Transfer
Treating a solution of cis-1-decalone with base causes an isomerization to take place. When the system reaches ...
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate ΔH° for the reaction using the given bond dissociation energies. CH4(g)+2O2(g)⟶CO2(g)+2H2O(g) Bond ΔH° (kJ/mol) O–OO–O 142 H–OH–O 459 C−HC−H 411 C=OC=O 799 O=OO=O 498 C–OC–O 358 What type of reaction is this?arrow_forwardCalculate AH for the reaction NH3 (g) + CH4 (g) → HCN (g) + 3 H2 (g) , from the following data. N2 (g) + 3 H2 (g) → 2 NH3 (g) ΔΗ- – 91. 8 kJ /mol C (s, graphite) + 2 H2 (g) → CH4 (g) - 74. 9 kJ / mol AH = 2 C (s, graphite) + H2 (g) + N2 (g) → 2 HCN (g) AH = ΔΗ: 270. 3 kJ / molarrow_forwardGiven the following combustion data for three different fuels: CH4 (g), C8H18 (l) and CH3OH (l),CH4 (g) + 2 O2 (g) → CO2 (g) + 2 H2O (l) ΔH° = −890 kJ2 C8H18 (l) + 25 O2 (g) → 16 CO2 (g) + 18 H2O (l); ΔH°= −10,914 kJ2 CH3OH (l) + 3 O2 (g) → 2 CO2 (g) + 4 H2O (l) ΔH° = −1453 kJwhich fuel provides the most energy per gram upon combustion and which provides the least? could you please explain this problemarrow_forward
- Use the following equations to calculate the heat of the reaction for the synthesis of diborane. 2B(s) + 3/2 O2(g) → B2O3(s) ∆ Ho = -1273 kJ B2H6(g) + 3 O2(g) → B2O3(s) + 3H2O(g) ∆ Ho = -2035 kJ H2(g) + 1/2 O2(g) → H2O(l) ∆ Ho = -286 kJ H2O (l) → H2O (g) Δ Ho = 44 kJ Calculate ∆Ho for the following reaction:2B(s) + 3H2(g) → B2H6(g)arrow_forwardGiven the following data: Br2(l) + 5F2(g) → 2BrF5(l) ΔH°=-918.0 kJ BrF3(l) + Br2(l) → 3BrF(g) ΔH°=125.2 kJ 2NaBr(s) + F2(g) → 2NaF(s) + Br2(l) ΔH°=-316.0 kJ NaBr(s) + F2(g) → NaF(s) + BrF(g) ΔH°=-216.6 kJ calculate ΔH° for the reaction:BrF3(l) + F2(g) → BrF5(l)ΔH°=arrow_forwardUse the indicated average bond enthalpies to estimate the change in enthalpy, ΔHo, for the reaction between methane and iodine to produce iodomethane and hydrogen iodide: CH4(g) + I2(g) → CH3I(g) + HI(g) ΔHo = ? It may be helpful to draw the Lewis electron dot structure for each reactant and product; all reactants and products have single bonds. average bond enthalpies (kJ) C - H 413 , C - I 240 , H - I 299 , I - I 151 Express your answer in units of kilojoules, but do not include the units on your submitted answer.arrow_forward
- A) Given the standard enthalpy changes for the following two reactions:(1) 2C(s) + H2(g)C2H2(g)...... ΔH° = 226.7 kJ(2) 2C(s) + 2H2(g)C2H4(g)......ΔH° = 52.3 kJwhat is the standard enthalpy change for the reaction:(3) C2H2(g) + H2(g)C2H4(g)......ΔH° = ?------- kJ B) Given the standard enthalpy changes for the following two reactions:(1) 2Pb(s) + O2(g)2PbO(s)...... ΔH° = -434.6 kJ(2) Pb(s) + Cl2(g)PbCl2(s)......ΔH° = -359.4 kJwhat is the standard enthalpy change for the reaction:(3) 2PbCl2(s) + O2(g)2PbO(s) + 2Cl2(g)......ΔH° = ?------ kJarrow_forwardFor the complete combustion of 1.000 mole of propane gas at 298 K and 1 atm pressure, ∆H° = -2220 kJ/mol. What will be the heat released when 4.13 g of propane is combusted under these conditions? The chemical formula for propane is C3H8.arrow_forward6H2O (l) + 6CO2 (g) → C6H12O6 (s) + 6O2 (g) ΔH = +2803 kJ/mol Calculate the amount of sunlight energy required (in kJ) by a plant to synthesize 75.0 g of glucose (C6H12O6).arrow_forward
- Calculate ΔH for the reaction: C2H4 (g) + H2 (g) → C2H6 (g), from the following data.arrow_forwardUsing the table of thermodynamic data provided, for the reaction below: CS₂(1) + 302(g) → CO2(g) + 2SO2(g) Compound CO₂(g) CS₂(1) O₂(g) SO₂(g) SO₂(g) AHºf (kJ/mol) -393.5 89.0 0 -296.8 -395.7 S°(J/K mol) 213.8 151.3 205.15 248.2 256.8 Determine/calculate and report the following values in the labelled boxes below: a) The standard enthalpy change (AH°, in kJ/mol) of the reaction. b) The standard entropy change (AS°, in J/K mol) of the reaction. c) The standard free energy change (AG°, in kJ/mol) of the reaction. d) The free energy change (AG, in kJ/mol) of the reaction at 7.50 x 10² K.arrow_forwardAssume that the complete combustion of one mole of glucose, a monosaccharide, to carbon dioxide and water liberates 2870 kJ of energy (AG°¹ = −2870 kJ/mol). If the energy generated by the combustion of glucose is entirely converted to the synthesis of a hypothetical compound X, calculate the number of moles of the compound that could theoretically be generated. Use the value AGo' compound X = -54.9 kJ/mol. Round your answer to two significant figures. moles: =arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY