
Interpretation:
For the given reaction under given conditions the balanced equation, conjugate acid-base pair and the direction of equilibrium should be identified.
Concept introduction:
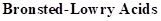 : If a species loses a proton then it is considered as
: If a species loses a proton then it is considered as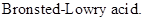
Strong Acids: Acids that dissociates into ions completely which results in easy donation of protons are considered as strong acids. Strong acid forms weaker conjugated base.
Weak Acids: Acids that do not easily dissociate into ions completely which has difficulty in proton donation are considered as weak acids. Weak acid forms stronger conjugated base
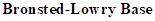 : If a species receives one proton, then it is considered as
: If a species receives one proton, then it is considered as 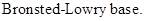
Strong Base: Bases that has strong attraction towards protons and accepts readily. Strong base forms weaker conjugated acid.
Weak Base: Bases that has little affinity towards protons. Weak base forms stronger conjugated acid.
If a base receives one proton, then the formed species is a conjugate acid whereas an acid lose one proton, then the formed species is a conjugated base.
Equilibrium: A state of a
The 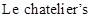 principle says that when a disturbance is given to equilibrium of a chemical reaction, the equilibrium will respond by shifting to the direction at which the effect of disturbance is nullified
principle says that when a disturbance is given to equilibrium of a chemical reaction, the equilibrium will respond by shifting to the direction at which the effect of disturbance is nullified
Balanced Chemical Reaction: On accordance with law of conservation of mass, for any chemical reaction, total masses of reactants and products must be equal.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Fundamentals of General, Organic, and Biological Chemistry (Principles of Chemistry - CHEM 1121)
- How is Ka defined? Write the equation for Ka for the generalized acid HA.arrow_forwardwhat is the conjugate acid for the following reaction? HC2HO4 + H2O <--> H3O+ + C2HO4- A)C2HO4- B) HC2HO4 C)H2O D) H3O+arrow_forwardGlucose-1-phosphate has a ΔG°′ value of −20.9 kJ/mol, whereas that for glucose-6-phosphate is −12.5 kJ/mol. After reviewing the molecular structures of these compounds, explain why there is such a difference in these values.arrow_forward
- The ΔG°′ value for glucose-1-phosphate is -20.9 kJ/mol. If glucose and phosphate are both at 4.8 mM, what is the equilibrium concentration of glucose-1-phosphate?arrow_forwardWrite a stepwise mechanism for the following reaction.arrow_forwardYou have an initial solution in which you added quantities of “A” and “B” such that there is 4.5 M “A” and 2.5 M “B” and no complex (“AB”) at time 0. After equilibrium, you are able to isolate and quantitate the “AB” complex, and find its concentration is 1.5 M. Given that RT is 0.59 kcal/mol, what is the delta Go’ for the association reaction?arrow_forward
- What is the ratio of [isocitrate] to [citrate] under cellular conditions at 37°C?arrow_forwardDraw the product AND propose a reasonable, detailed stepwise mechanism, using curved arrow notation to show the flow of electrons, for the following reaction.arrow_forwardIf glucose, phosphate, and glucose-6-phosphate arecombined in concentrations of 4.8, 4.8, and 0.25 mM,respectively, what is the equilibrium constant for thehydrolysis of glucose-6-phosphate at a temprature of258C?arrow_forward
- Based on the Henderson-Hasselbalch equation (shown below), calculate the pH when half of a solution of acetic acid is dissociated to acetate (the pKa of acetic acid is 4.76). A. 1.00 B. 3.76 C. 4.76 D. 5.76arrow_forwardPlease calculate the melting temperatures of oligomer A&B by the equation provided in the photoarrow_forwardwhich statement is true for the following reaction?arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





