
(a)
Interpretation:
Whether the alloy is hypoeutectic or hypereutectic needs to be determined.
Concept Introduction:
Eutectic alloy is defined as a mixture of metals having a melting point lower than that of any of components. An alloy which has composition that lies to the left of the eutectic point present on the phase diagram is termed as hypoeutectic alloy. The hyper-eutectic alloy is defined as the alloy which consist of composition that lies to the extreme right of the eutectic point.
Answer to Problem 11.31P
The Ai-
Explanation of Solution
The Al-
Therefore, Al-
The hyper-eutectic alloy is defined as the alloy which consist of composition that lies to the extreme right of the eutectic point.
Hence, as per the hyper-eutectic alloy conditions, the given Ai-
(b)
Interpretation:
The value of composition of the first solid formed during the solidification process needs to be determined.
Concept Introduction:
Solidification is the method which is also known as freezing process. Solidification process is defined as the phase change of matters. The phase change of matter that results in the production of solid phase. Regularly, this occurs when the temperature of the liquid is lowered below the freezing point. Undercooling of liquid takes place in the process of solidification. Solidification can yield metastable product structures at high undercooling. The constituents of the metastable products are the result of kinetic competition.
Answer to Problem 11.31P
The value of composition is
Explanation of Solution
During solidification the first solid formed has some amount of composition. The value of composition of the first solid formed during solidification of Al-
Therefore, composition where the first solid phase occurs as
(c)
Interpretation:
The amount and composition of each phase of temperature of
Concept Introduction:
Solidification is the method which is also known as freezing process. Solidification process is defined as the phase change of matters. The phase change of matter that results in the production of solid phase. Regularly, this occurs when the temperature of the liquid is lowered below the freezing point. Undercooling of liquid takes place in the process of solidification. Solidification can yield metastable product structures at high undercooling. The constituents of the metastable products are the result of kinetic competition.
Answer to Problem 11.31P
The amount of the phase obtained is
Explanation of Solution
The given temperature is
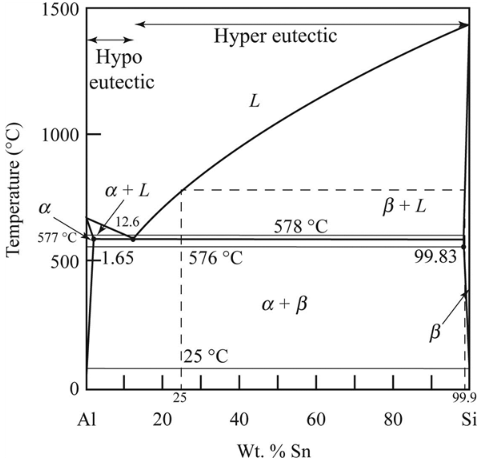
So, we can calculate the weight percentage of eutectic phase
Thus, we obtained the amount and composition of phase from the above standard phase diagram as
(d)
Interpretation:
The amount and composition of each phase at temperature of
Concept Introduction:
Solidification is the method which is also known as freezing process. Solidification process is defined as the phase change of matters. The phase change of matter that results in the production of solid phase. Regularly, this occurs when the temperature of the liquid is lowered below the freezing point. Undercooling of liquid takes place in the process of solidification. Solidification can yield metastable product structures at high undercooling. The constituents of the metastable products are the result of kinetic competition.
Answer to Problem 11.31P
The amount of phase obtained is
Explanation of Solution
The given temperature is
The amount of each phase at
To calculate the composition of each phase at
To calculate the composition of phase, we have the expression,
So, we can calculate the weight percentage of eutectic phase
(e)
Interpretation:
The amount and composition of each micro constituent at the temperature of
Concept Introduction:
Solidification is the method which is also known as freezing process. Solidification process is defined as the phase change of matters. The phase change of matter that results in the production of solid phase. Regularly, this occurs when the temperature of the liquid is lowered below the freezing point. Undercooling of liquid takes place in the process of solidification. Solidification can yield metastable product structures high undercooling. The constituents of the metastable products are the result of kinetic competition.
Answer to Problem 11.31P
The amount of eutectic phase and phase is calculated at
Explanation of Solution
The phases present at eutectic phase and phase is calculated as
The phases present at the
From the diagram mentioned above at
To calculate the composition of eutectic phase at
To calculate the composition of primary phase −
So, the weight percentage of eutectic phase
(f)
Interpretation:
The amount and composition of each phase at
Concept Introduction:
Solidification is the method which is also known as freezing process. Solidification process is defined as the phase change of matters. The phase change of matter that results in the production of solid phase. Regularly, this occurs when the temperature of the liquid is lowered below the freezing point. Undercooling of liquid takes place in the process of solidification. Solidification can yield metastable product structures at high undercooling. The constituents of the metastable products are the result of kinetic competition.
Answer to Problem 11.31P
The number of phases present in Al-
Explanation of Solution
The phases present at
From the diagram mentioned above the amount of the phases calculated are
To calculate the composition of phase at
So, one can calculate the weight percentage of eutectic phase
Want to see more full solutions like this?
Chapter 11 Solutions
Essentials Of Materials Science And Engineering
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





