
(a)
Interpretation:
State that the copper element A or element B is labeled in given phase diagram of copper-silver metals.
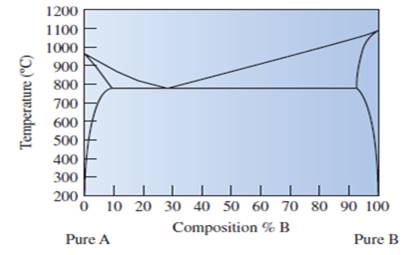
Fig.
Concept Introduction:
The properties of the copper-silver alloy are as follows-
1. Melting point −
2. Molecular weight −
3. Density −
4. Insoluble in water
In the case of silver and copper alloy, both of these are not able to melt together and form a new alloy with
1. Sterling silver − In sterling silver, the weight percentage of pure silver is
2. Coin silver - The weight percentage of pure silver available in coin silver is
There is a new alloy also available known as Electrum. In this alloy, the weight percentage of silver is from
(b)
Interpretation:
The well labelled phase diagram of the copper silver alloy needs to be sketched.
Concept Introduction:
The properties of the copper-silver alloy are as follows-
1. Melting point −
2. Molecular weight −
3. Density −
4. Insoluble in water
In the case of silver and copper alloy, both of these are not able to melt together and form a new alloy with
1. Sterling silver − In sterling silver, the weight percentage of pure silver is
2. Coin silver - The weight percentage of pure silver available in coin silver is
There is a new alloy also available known as Electrum. In this alloy, the weight percentage of silver is from
(c)
Interpretation:
Whether the new composition is stronger or weaker needs to be determined, if it cools down at
Concept Introduction:
The properties of the copper-silver alloy are as follows-
1. Melting point −
2. Molecular weight −
3. Density −
4. Insoluble in water
In the case of silver and copper alloy, both of these are not able to melt together and form a new alloy with
1. Sterling silver − In sterling silver, the weight percentage of pure silver is
2. Coin silver - The weight percentage of pure silver available in coin silver is
There is a new alloy also available known as Electrum. In this alloy, the weight percentage of silver is from
(d)
Interpretation:
The statement "microstructure can lead to the discrepancy" needs to be justified by giving an example.
Concept Introduction:
The properties of the copper-silver alloy are as follows-
1. Melting point −
2. Molecular weight −
3. Density −
4. Insoluble in water
In the case of silver and copper alloy, both of these are not able to melt together and form a new alloy with
1. Sterling silver − In sterling silver, the weight percentage of pure silver is
2. Coin silver - The weight percentage of pure silver available in coin silver is
There is a new alloy also available known as Electrum. In this alloy, the weight percentage of silver is from
Trending nowThis is a popular solution!

Chapter 11 Solutions
Essentials Of Materials Science And Engineering
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





