
Answer the following questions about levonorgestrel (trade name Plan B). Levonorgestrel interferes with ovulation, the release of an egg from an ovary, so it prevents pregnancy if taken within a few days of unprotected sex.
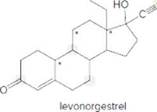
- Identify the
functional groups . - How many C’s does levonorgestrel contain?
- How many H’s are present at each C labeled with an asterisk (*)?
- Give the shape around each atom labeled in gray.
- Label all polar bonds.
- Is levonorgestrel soluble in an organic solvent?
- Is levonorgestrel soluble in water?
(a)
Interpretation:
The functional group in levonorgestral should be identified.
Concept Introduction:
Functional groups are the atoms or group of atoms that gives chemical properties to an organic compound and also chemical reactivity centre. Examples of functional groups are aldehyde, ketone, alcohol, alkene, alkyne etc.
Answer to Problem 84P
The functional groups present in the compound are alcohol, ketone, alkene and alkyne.
Explanation of Solution
Functional groups are the atoms or group of atoms that gives chemical properties to an organic compound and also chemical reactivity centre. Structures of different functional groups are as follows:
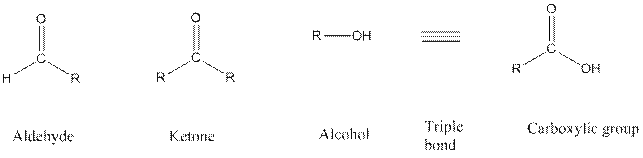
In the above functional group R represents an alkyl group.
In functional group ketone carbonyl carbon is bonded to two alkyl groups, in aldehyde carbonyl carbon is attached to one alkyl group and one hydrogen atom, in alcohol functional group an −OH is bonded to an alkyl group, in alkyne there is a triple bond between two carbon atoms and in alkene there is a double bond between two carbon atoms.
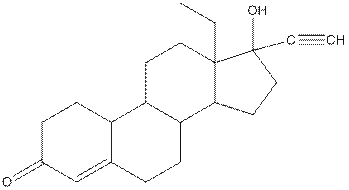
Levonorgestral structure is as follows:
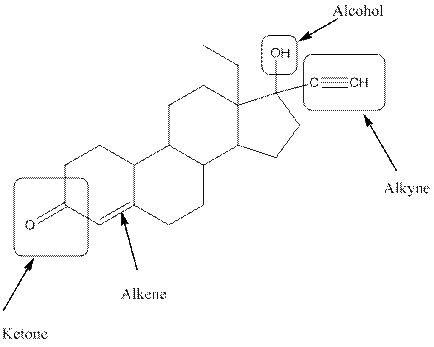
Observe the structure of Levonorgestral. As indicated in the above figure, four types of functional groups present that is, alkene, alkyne, alcohol and ketone. The functional groups present in the compound are alcohol, ketone, alkene and alkyne.
(b)
Interpretation:
The number of carbon atoms in the structure of levonorgestral should be determined.
Concept Introduction:
In skeletal structure the terminals represent methyl
Answer to Problem 84P
Twenty one carbon atoms present in the compound.
Explanation of Solution
The structure of Levonorgestral is as follows:
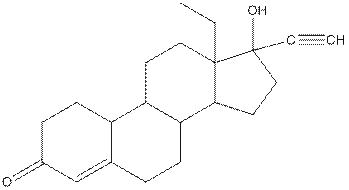
In skeletal structure the terminals represent methyl
Hence, twenty one carbon atoms present in the compound.
(c)
Interpretation:
The number of hydrogen atoms present in the * carbon atoms in levonorgestral should be determined.
Concept Introduction:
In skeletal structure the terminals represent methyl
Answer to Problem 84P
Number of hydrogen atoms in * carbon atoms are three.
Explanation of Solution
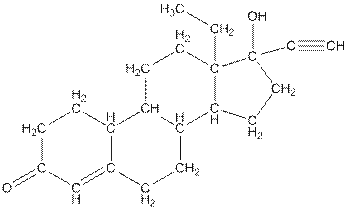
Complete structure of levonorgestral is as follows:
From the complete structure of Levonorgestral, it is clear that number of hydrogen atoms in * carbon atoms are three.
(d)
Interpretation:
The shape around each indicated carbon atom in levonorgestral should be determined.
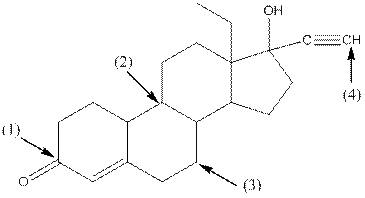
Concept Introduction:
The following table should be used while determining the shape around an atom.
| Number of groups | Number of atoms | Number of lone pairs | Shape | Bond angle |
| 2 | 2 | 0 | Linear | |
| 3 | 3 | 0 | Trigonal planar | |
| 4 | 4 | 0 | Tetrahedral | |
| 4 | 3 | 1 | Trigonal pyramidal | |
| 4 | 2 | 2 | Bent |
Answer to Problem 84P
Shape around carbon 1 is trigonal planar, shape around carbon 2 is tetrahedral, shape around carbon 3 is tetrahedral and shape around carbon 4 is linear.
Explanation of Solution
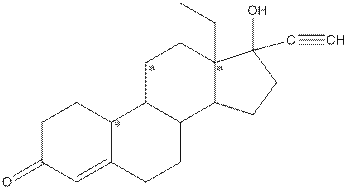
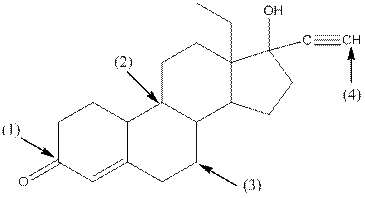
Given compound is as follows:
Complete structure of the compound is as follows:
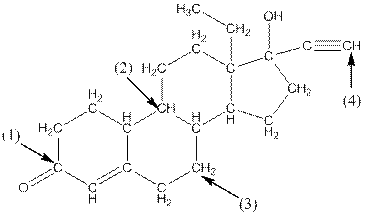
- Around C1, three atoms (two carbon and one oxygen) present. So, shape around carbon 1 is trigonal planar.
- Around C2, four atoms (three carbon and one hydrogen) present. So, shape around carbon 2 is tetrahedral.
- Around C3, four atoms (two carbon and two hydrogen) present. So, shape around carbon 3 is tetrahedral.
- Around C4, two atoms ( one carbon and one hydrogen) present. So, shape around carbon 4 is linear.
(e)
Interpretation:
All the polar bonds in levonorgestral should be labeled.
Concept Introduction:
The unequal sharing of valence electrons in a bond is called polar bond. Polar bond result when the bond formed between two atoms in which one atom is more electronegative than the other one. One example of polar bond is
Structure of HCl is as follows:

In
Answer to Problem 84P
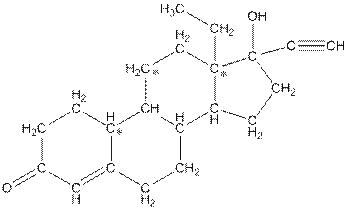
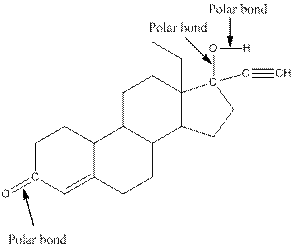
The structure of levonorgestral with labeled all polar bonds is as follows:
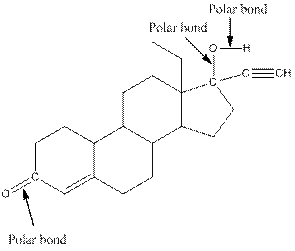
Explanation of Solution
In organic compound, most of the polar bonds formed between carbon and heteroatoms like oxygen, nitrogen, sulphur etc.
In the compound, three polar bonds present. One polar bond is between carbon and oxygen where oxygen is more electronegative than carbon. Second polar bond is between carbon and oxygen where oxygen is more electronegative than carbon and third polar bond is between oxygen and hydrogen where oxygen is more electronegative than hydrogen.
The structure of levonorgestral with labeled all polar bonds is as follows:
(f)
Interpretation:
Whether levonorgestral is soluble in organic solvent or not should be determined.
Concept Introduction:
A compound is soluble in organic solvent if the compound contains too many hydrocarbon bonds that is if it is non-polar.
Answer to Problem 84P
The compound levonorgestral will soluble in organic solvent.
Explanation of Solution
The complete structure of levonorgestral is as follows:
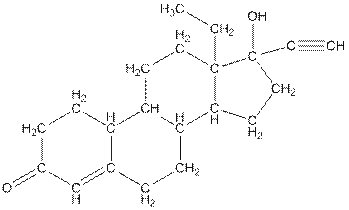
The compound levonorgestral has too many carbon hydrogen bonds. So, the compound will soluble in organic solvent.
(g)
Interpretation:
Whether levonorgestral is soluble in water or not should be determined.
Concept Introduction:
Water is a polar solvent. To dissolve in water the compound must be polar. Polar compound is that compound in which polar bonds are present. The unequal sharing of valence electrons in a bond is called polar bond. Polar bond result when the bond formed between two atoms in which one atom is more electronegative than the other one. One example of polar bond is
Structure of HCl is as follows:

In
Answer to Problem 84P
The compound levonorgestral is water soluble.
Explanation of Solution
Structure of levonorgestral is as follows:
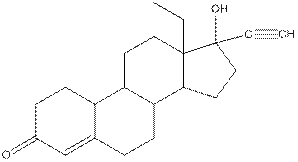
In the compound levonorgestral, two oxygen heteroatoms present. These heteroatoms can form hydrogen bonds with water molecule and hence, the compound is soluble in water.
Want to see more full solutions like this?
Chapter 11 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Using the following guidelines, draw four constitutional isomers from this chemical formula: C6H8O. Each isomer must meet the structural requirement listed. a. Draw a constitutional isomer that contains only one ring b. Draw a constitutional isomer that contains at least one triple bond c. Draw a constitutional isomer that contains at least one alcohol functional group d. Draw a constitutional isomer that contains at least one ketone functional grouparrow_forwardBased on the photo, Identify the functional group that appears in the following molecule that is known as cadaverine because of its first isolation from cadavers. A. Nitrile B. Ketone C. Amide D. Aldehyde E. Aminearrow_forwardch-1 Dont provide handwriting solutionarrow_forward
- identify the functional group of each compound.arrow_forwardCH3CH2OH CH3SH CH3CH2CH3 Which compound can form hydrogen bonds? Which compound has the highest boiling point? Which compound has the lowest boiling point?arrow_forwardrank the following compounds in order of decreasing boiling point with 1 having the highest boiling point and 3 having the lowest boiling point. CH3CH2Cl, CH3CCL3, CH3OHarrow_forward
- The skeletal formula represents what type of alcohol? primary secondary tertiary quaternary none QUESTION 2 Thiols are strong- smelling compounds responsible for ________. skunky or bad smelling odors flowery odors sharp odors salty odors fruity odors QUESTION 3 The skeletal formula represents what type of alcohol? primary secondary tertiary quaternary none QUESTION 4 A phenol has an - OH group bonded to a(n) ________. tetrasubstituted carbon disubstituted carbon singly substituted or unsubstituted carbon trisubstituted carbon carbon. in a benzene ring QUESTION 5 The common name of CH 3— CH 2— O— CH 2— CH 3 is ________. 2-etherbutane diethyl ether dimethyl ether dibutyl ether butyl ether QUESTION 6 In the IUPAC naming system, a ketone is named by replacing the - e in the corresponding alkane name with ________. al ol one ene yne QUESTION 7 When a primary alcohol is strongly oxidized, the product is ________. an aldehyde an alkane another alcohol a carboxylic acid a…arrow_forwardFor each compound,(1) classify the nitrogen-containing functional groups.(2) provide an acceptable name.CH3 CCH3CH3CH2 NH2(a) CH3CHCH3NHCH3arrow_forwardAmyl nitrite contains the following functional group: Select one: a. -NO2 b. -C=N c. -ONO d. -ONO2arrow_forward
- Which of the following statements about acetone is/are correct?I. Large amount of acetones are produced in the human body.II. Diabetic patients produces larger amounts of acetone.III. In severe diabetes, odor of acetone can be detected on the person's breath.IV. Acetone is partially a by-product in gasoline treatments designed for engine immiscibility to water.arrow_forwardHow GC-MS separates and identify organic compounds?arrow_forward5. a) Draw two different structures with the molecular formula C2H6O. b) Name the functional group in each structure c)Which one will have the higher boiling point, and whyarrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

