
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1.19B, Problem 1.25P
Give the relationship between the following pairs of structures The possible relationships are
| same compound | constitutional isomers (structural isomers) |
| Cis-trans isomers | not isomers (different molecular formula) |

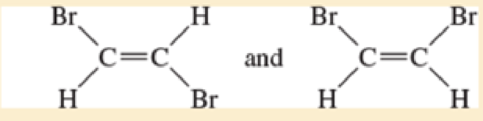
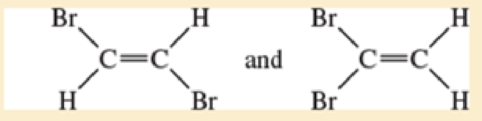
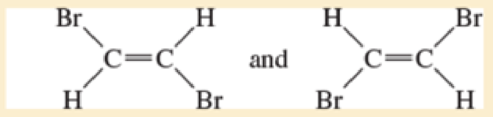
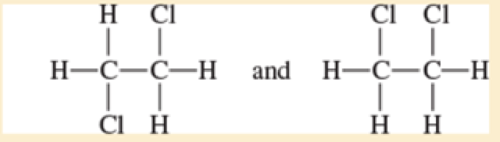
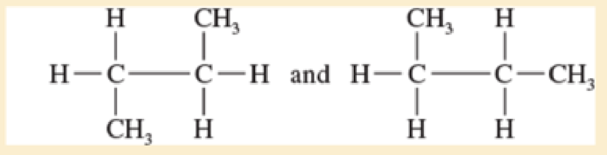
- a. CH3—CH2— CH2— CH3 and CH3—CH=CH—CH3
- b. CH:=CH—CH2CH2CH3 and CH3—CH=CH—CH2CH
- c. CH2=CHCH2CH2CH3 and CH3CH2CH2CH=CH2
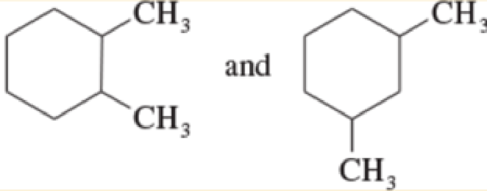
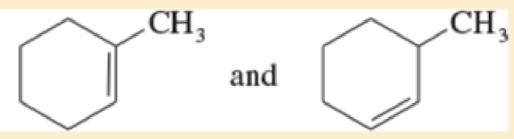
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Complete the following molecular formula of Ethyl acrylate with C, CH, CH2, or CH3.
1 Which of the following has(have) the same molecular formula as hexane? A. 3-ethylpentane
B. 2,4-dimethylpentane
C. 3,3-dimethylpentane
D. 2,3-dimethylpentane
E. 2,3-dimethylbutane
1b.
Which of these formulas is(are) not theoretically possible? A. C6H6
B. C6H10 C. C6H12 D. C6H14 E. C6H16
1c.
Which of these formulas is(are) not valid for hexane?
A. C6H14
B. C6H6C. C6H16D. CH3CH2CH2CH2CH2CH3
E. CH3(CH2)4CH3
How many isomers, both structural and geometric, have the molecular formula C5H12?
Chapter 1 Solutions
Organic Chemistry (9th Edition)
Ch. 1.2C - a. Nitrogen has relatively stable isotopes...Ch. 1.4 - Draw Lewis structures for the following compounds....Ch. 1.5 - Write Lewis structures for the following molecular...Ch. 1.5 - Circle any lone pairs (pairs of nonbonding...Ch. 1.6 - Use electronegativities to predict the direction...Ch. 1.8 - Prob. 1.6PCh. 1.9B - Draw the important resonance forms for the...Ch. 1.9B - Prob. 1.8PCh. 1.9B - Prob. 1.9PCh. 1.9B - Use resonance structures to identify the areas of...
Ch. 1.10A - Draw complete Lewis structures for the following...Ch. 1.10B - Give Lewis structures corresponding to the...Ch. 1.10B - Prob. 1.13PCh. 1.11 - Compute the empirical and molecular formulas for...Ch. 1.16 - a. Use your molecular models to make ethane, and...Ch. 1.17 - a. Predict the hybridization of the oxygen atom in...Ch. 1.17 - Predict the hybridization geometry and bond angles...Ch. 1.17 - Predict the hybridization, geometry, and bond...Ch. 1.17 - Prob. 1.19PCh. 1.17 - Allene, CH2=C=CH2, has the structure shown below...Ch. 1.17 - 1. Draw the important resonance forms for each...Ch. 1.18B - Prob. 1.22PCh. 1.18B - Two compounds with the formula CH3CH=NCH3 are...Ch. 1.19B - Prob. 1.24PCh. 1.19B - Give the relationship between the following pairs...Ch. 1 - a. Draw the resonance forms for SO2 (bonded OSO)....Ch. 1 - Name the element that corresponds to each...Ch. 1 - Prob. 1.28SPCh. 1 - For each compound, state whether its bonding is...Ch. 1 - a. Both PCl3 and PCl5 are stable compounds Draw...Ch. 1 - Draw a Lewis structure for each species a. N2H4 b....Ch. 1 - Prob. 1.32SPCh. 1 - Prob. 1.33SPCh. 1 - Draw Lewis structures for a. two compounds of...Ch. 1 - Prob. 1.35SPCh. 1 - Some of the following molecular formulas...Ch. 1 - Prob. 1.37SPCh. 1 - Give the molecular formula of each compound shown...Ch. 1 - 1. From what you remember of electronegativities,...Ch. 1 - For each of the following structures, 1. Draw a...Ch. 1 - Prob. 1.41SPCh. 1 - Prob. 1.42SPCh. 1 - Prob. 1.43SPCh. 1 - Prob. 1.44SPCh. 1 - For each pair of ions, determine which on is more...Ch. 1 - Use resonance structures to identify the areas of...Ch. 1 - Prob. 1.47SPCh. 1 - In 1934, Edward A. Doisy of Washington University...Ch. 1 - If the carbon atom in CH2Cl2 were fat. there would...Ch. 1 - Cyclopropane (C3H6, a three-membered ring) is more...Ch. 1 - Prob. 1.51SPCh. 1 - Prob. 1.52SPCh. 1 - In most amines, the nitrogen atom is sp3...Ch. 1 - Predict the hybridization and geometry of the...Ch. 1 - Draw orbital pictures of the pi bonding in the...Ch. 1 - Prob. 1.56SPCh. 1 - Prob. 1.57SPCh. 1 - Which of the following compounds show cis-trans...Ch. 1 - Give the relationships between the following pairs...Ch. 1 - Dimethyl sulfoxide (DMSO) has been used as an...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many electron pairs are shared when a triple bond exists between two carbon atoms? What must he the geometric arrangement around the carbon atoms in a triple bond? Draw the Lewis structure of a simple molecule that contains a triple bond.arrow_forwardMatch the compound name with its correct molecular formula (based on the general formula): - propane - propene - butene - butyne A. C4H8 B. C4H6 C. C3H8 D. C3H6 Sent by you: Match the compound name with its correct molecular formula (based on the general formula): - propane - propene - butene - butyne A. C4H8 B. C4H6 C. C3H8 D. C3H6arrow_forwardThe structures below are: A) not isomers.B) conformational isomers.C) cis-trans isomers.D) structural isomers.E) both B and Darrow_forward
- 1. Considering compounds that have the same number of carbon atoms, explain why alkanes and cycloalkanes have different molecular formulas but alkenes and cycloalkanes have the same molecular formulas. 2. Draw the condensed or line-angle structure for an alkene with the formula C5H10. 3. Draw the condensed or line-angle structure for 4 more C5H10 isomers (2 additional alkenes and 2 cyclic isomers).arrow_forwardIndustrial Chemistry: Rank the following molecules in INCREASING order of octane rating, Octane, Isooctane, propane, methane, 2-methylpropanearrow_forwardOrganic compounds may have characteristic odors as well as other characteristic physical properties. For example, the distinct odor of the seashore at low tide results in part from the presence of dimethyl sulfide (CH3SCH3), a molecule with a similar structure to dimethyl ether (CH3OCH3). Ethanethiol (CH3CH2SH), also called mercaptan, is an isomer of dimethyl sulfide with a much less pleasant odor.The table lists four related compounds and their enthalpies of vaporization (ΔH°vap) in kJ/mol. Compound ΔH°vap (kJ/mol) CH3OCH3 23 CH3SCH3 28 CH3CH2SH 27.5 CH3CH2OH 42 Rank the following compounds in order of increasing strength of their intermolecular forces, given the ΔH°vap listed for each. Place the compound with the strongest intermolecular forces (IMFs) at the top of the list. (Strongest to weaknest). Why is ΔHºvap for CH3SCH3 greater than ΔHºvap for CH3OCH3? A. CH3OCH3 is more polar. B. CH3SCH3 has stronger dipole–dipole attractions. C. CH3OCH3 can form…arrow_forward
- 1. Describe how geometric isomers are different from structural isomers. 2. Why is it possible for alkenes and cylcoalkanes to have cis-trans isomers but not alkanes? 3. How are cis-trans isomers shown in diagrams?arrow_forwardWrite structural formulas for all the constitutional isomers of (a) C3H8 (b) C3H6 (c) C3H4arrow_forwardDraw an acceptable Lewis structure from each condensed structure, such that all atoms have zero formal charge. a. diethyl ether, (CH3CH2)2O, the first general anesthetic used in medical procedures b.acrylonitrile, CH2CHCN, starting material used to manufacture synthetic Orlon fibers c.dihydroxyacetone, (HOCH2)2CO, an ingredient in sunless tanning products d.acetic anhydride, (CH3CO)2O, a reagent used to synthesize aspirinarrow_forward
- Draw an acceptable Lewis structure from each condensed structure, such that all atoms have zero formal charge. a diethyl ether, (CH3CH2)2O, the first general anesthetic used in medical procedures b. acrylonitrile, CH2CHCN, starting material used to manufacture synthetic Orlon fibers c.dihydroxyacetone, (HOCH2)2CO, an ingredient in sunless tanning products d.acetic anhydride, (CH3CO)2O, a reagent used to synthesize aspirinarrow_forwardDraw an acceptable Lewis structure from each condensed structure, such that all atoms have zero formal charge. a. diethyl ether, (CH3CH2)2O, the first general anesthetic used in medical procedures b. acrylonitrile, CH2CHCN, starting material used to manufacture synthetic Orlon fibers c. dihydroxyacetone, (HOCH2)2CO, an ingredient in sunless tanning products d. acetic anhydride, (CH3CO)2O, a reagent used to synthesize aspirinarrow_forwardA. What is the abbreviated structural and skeletal formulas for the linear hydrocarbon with the molecular formula C4H10? B. During a chemical reaction, one bromine atom replaces one of the hydrogen atoms in the compound above. Draw the skeletal formula for all the possible isomers that could be formed as a result of this replacement.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License