
Concept explainers
12-27 Explain why each name is incorrect and then write a correct name.
- 2-Ethyl-l-propene
- 5-lsopropylcyclohexene
- 4-Methyl-4-hexene
- 2-sec-Butyl-l-butene
- 6,6-Dimethylcyclohexene
- 2-Ethyl-2-hexene
(a)
Interpretation:
To identify the error in name of the given compound and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 12.27P
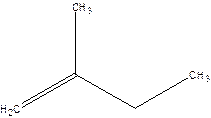
2-Methyl-but-1-ene.
Explanation of Solution
2-Ethyl-1-propene.
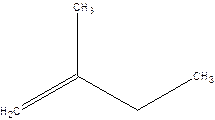
Error- longest chain not correctly located.
Correct name-
2-Methyl-but-1-ene.
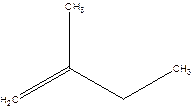
(b)
Interpretation:
To identify the error in name of the given compound and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 12.27P
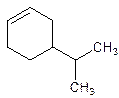
4-Isopropylcyclohexene.
Explanation of Solution
5-Isopropylcyclohexene.
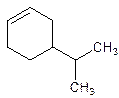
Error- position of the substituent not correctly mentioned.
Correct name-
4-Isopropylcyclohexene.
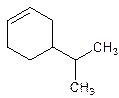
(c)
Interpretation:
To identify the error in name of the given compound and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 12.27P

3-Methyl-2-hexene.
Explanation of Solution
4-Methyl-4-hexene.
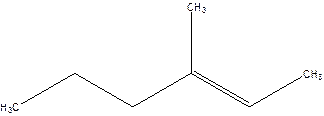
Error- position of the double bond not correctly mentioned.
Correct name-
3-Methyl-2-hexene.
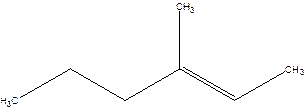
(d)
Interpretation:
To identify the error in name of the given compound and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 12.27P
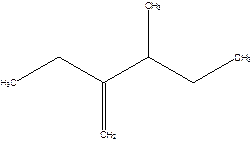
2-Ethyl-3-methyl -1-pentene.
Explanation of Solution
2-sec-butyl-1-butene.
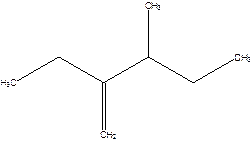
Error- longest chain not correctly determined.
Correct name-
2-Ethyl-3-methyl -1-pentene.
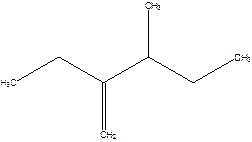
(e)
Interpretation:
To identify the error in name of the given compound and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 12.27P
3,3-Dimethylcyclohexene.
Explanation of Solution
6,6-Dimethylcyclohexene.
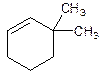
Error- position of substituents not correctly determined.
Correct name-
3,3-Dimethylcyclohexene.
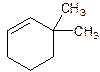
(f)
Interpretation:
To identify the error in name of the given compound and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 12.27P

3-Methyl-3-Heptene.
Explanation of Solution
2-Ethyl-2-hexene.

Error- longest chain not correctly determined.
Correct name-
3-Methyl-3-Heptene.

Want to see more full solutions like this?
Chapter 12 Solutions
Introduction to General, Organic and Biochemistry
- 12-23 Draw a structural formula for each compound. 3-Chloropropene 3-Methylcyclohexene 1,2-Dimethylcyclohexene /runs-3,4-Dimethyl-3-heptene Cydopropene 3-Hexynearrow_forward12-45 Draw a structural formula for the product of each reaction. 1-Methylcyclohexene + Br2 1,2-Dimethylcyclopentene + Cl2arrow_forward12-29 Which of these alkenes show cis-trans isomerism? For each that does, draw structural formulas for both isomers. (a) 1-Hexene(b)2-Hexene (c) 3-Hexene(d)2-Methyl-2-hexene (e) 3-Methyl-2-hexene(f)2,3-Dimethyl-2-hexenearrow_forward
- Draw structural formulas for all possible carbocations formed by the reaction of each alkene with HC1. Label each carbocation as primary, secondary, or tertiary. ch3 I ch3ch,c=chch3 ch3ch2ch=chch3arrow_forward12-50 Draw the structural formula of an alkene that undergoes acid-catalyzed hydration to give the indicated alcohol as the major product. More than one alkene may give each alcohol as the major product. 3-Hexanol 1-Methylcyclobutanol 2-Methyl-2-butanol 2-Propanolarrow_forwardName and draw structural formulas for all pos sible monochlorination products that might be formed in each reaction.arrow_forward
- 12-58 Show how to convert ethylene to these compounds (a) Ethane(b) Ethanol (c) Bromoethane(d) 1,2-Dibromoethane (e) Chloroethanearrow_forwardDraw the structural formula of an alkene that undergoes acid-catalyzed hydration to give each of the following alcohols as the major product. More than one alkene may give each compound as the major productarrow_forward12-40 Define alkene addition reaction. Write an equation for an addition reaction of propene.arrow_forward
- 13-53 Write the structural formula for the product of each reaction.arrow_forward12-77 Show how to convert cyclopentene into these compounds. 1,2-Dibromocyclopentane Cyclopentanol Iodocyclopentane Cyclopentanearrow_forward11-38 Why is equatorial methylcyclohexane more stable than axial methylcyclohexane?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
