
Concept explainers
(a)
Interpretation:
The following pair of molecules should be classified as constitutional isomers or identical molecules or not isomers of each other:
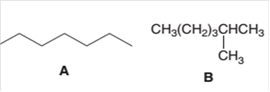
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds is
The molecular formula of an organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with
(b)
Interpretation:
The following pair of molecules should be classified as constitutional isomers or identical molecules or not isomers of each other:
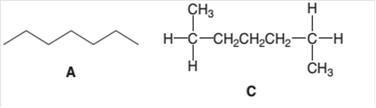
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds is organic chemistry.
The molecular formula of an organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule. Constitutional isomers can be defined as the pair of molecules with the same molecular formula but different structural formula.
(c)
Interpretation:
The following pair of molecules should be classified as constitutional isomers or identical molecules or not isomers of each other:
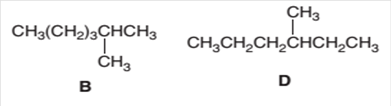
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds is organic chemistry.
The molecular formula of an organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule. Constitutional isomers can be defined as the pair of molecules with the same molecular formula but different structural formula.
(d)
Interpretation:
The following pair of molecules should be classified as constitutional isomers or identical molecules or not isomers of each other:
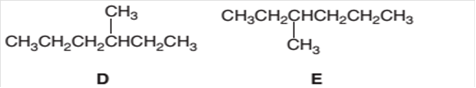
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds is organic chemistry.
The molecular formula of an organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule. Constitutional isomers can be defined as the pair of molecules with the same molecular formula but different structural formula.
(e)
Interpretation:
The following pair of molecules should be classified as constitutional isomers or identical molecules or not isomers of each other:
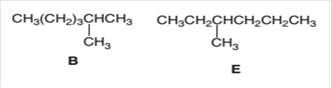
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds is organic chemistry.
The molecular formula of an organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule. Constitutional isomers can an as the pair of molecules with the same molecular formula but different structural formula.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
- The structures below are H. H CH₂ H H CH₂ A) not isomers B) conformational isomers C) cis-trans isomers D) structural isomers E) both B and D H H H CH3 H CH3arrow_forwardFor each of the following compounds and ions,1. Draw a Lewis structure.2. Show the kinds of orbitals that overlap to form each bond.3. Give approximate bond angles around each atom except hydrogen.(a) [NH2]- (b) [CH2OH]+ (c) CH2“N¬CH3(d) CH3¬CH“CH2 (e) HC‚C¬CHO (f) H2N¬CH2¬CNarrow_forwardPart A What is the relationship between the following molecules? CH,CH3 CH3 CH2 CHCH3 CH;CH2-CH- CH-CH,-CH-CH-CH3 CH3 CH,CH3 CH3 CHCH3 CH,CH3 CH;CH, CH-CH-CH,-CH–ĊH-CH,CH,CH3 CH3 CH3 O They are different molecules which are not isomers. O They are identical. O They are isomers of each other. O none of the above Submit Request Answerarrow_forward
- 12. Which of the following pairs of compounds are constitutional isomers? A) B) C) D) CH3CH₂OCH₂CH3 CI- Br CH 3 CH 3 CH3 H -H CH 3 CH3CH2CH2CH3 N-CH₂-OH and and CH3CH2CH₂OH and Br CI- CH 3 H -H CH 3 and (CH3)2CHCH2CH3 CH3CH₂-O-CH₂-NH2arrow_forwardWhich of the following pairs of compounds represent constitutional isomers? (1) H, and CI H (2) CH3- -CH3 and CH3-CH2- H. (3) H. CH3 CH3 CH3 H H- -CH3 CH- -CH2- H- and CH3- CH C- H- CH3CH2 CH3 (4) CH3. N-CH3 CH and CH3-N(CH3)2arrow_forward32 eBook Print References Select all constitutional isomers having the molecular formula C4H₁00. A E I ³X OH OH B F J O OH C G K OH D H OH lom OH Larrow_forward
- For the molecules shown below, which ones represent constitutional isomers of each other? OB and D O B, D, and E QE and F OD and F O A and C (CH₂),CCH₂CH₂CH(CH JCH, D . E H₂C H₂C CH₂ CH₂ CH CH₂ CH₂ hparrow_forwardQ6.7 B and D CH3 „CH3 H3C, CH3 CH3 CH3 H;C H3C" CH3 CH3 CH3 H;C B D F What is the relationship between molecules B and D? O They are the same molecule O They are enantiomers of one another O They are diastereomers of one another O They are constitutional isomers O There's no relationship between them, they have completely different chemical formulas Q6.8 B and E CH3 CH3 H3C,, CH3 CH3 H3C" CH3 CH; CH3 H;C CH3 D. E F What is the relationship between molecules B and E? O They are the same molecule O They are enantiomers of one another O They are diastereomers of one another O They are constitutional isomers O There's no relationship between them, they have completely different chemical formulasarrow_forwardCompound 1 Compound 3 Choose the two molecules that are constitutional isomers. Compound 2 Compound 4 A) Compounds 1 and 2 B) Compounds 1 and 3 Compounds 2 and 3 D) Compounds 2 and 4 E) Compounds 3 and 4arrow_forward
- 7. The following six compounds have a molecular formula of C5H₁0O. Give the relationship of each pair of molecules. Your options are geometric isomers, constitutional isomers, or the same molecule. H3C-O H3C A A and B: B and C: B and F: D and E: F and A: CH3 H3C-O H3C CH3 C CH3 H3C CH3 OH CH3 E CH3 H3C CH3 H3C Farrow_forwardXX. CH₂ H. H Label each pair of compounds below as: تے H₂C a. conformational isomers b. configurational isomers constitutional isomers identical not isomers C. d. e. CH3 CH 3 CH 3 and CI H CH3 and CH3 H H₂C- Н. H H H3C12 CH₂ CH3 CH3 CH₂ CI H3C- * The same letter can be use more than once!arrow_forwardCompound A Compound F HCI CH3OH H₂SO4 Compound B CH3 CH₂ CH₂ Cl Compound E NaOH K₂Cr₂O7 H₂SO4 Compound C K₂Cr₂O7 H₂SO4 Compound D a. Draw the structural formulas of compounds A, C, D, E and F in the boxes provided above.arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
