
Concept explainers
a)
Interpretation:
The possible products containing only one bromine has to be predicted.
Concept Introduction:
Free radical stability: As the number of carbon substituents increases, the stability of the free radical increases.
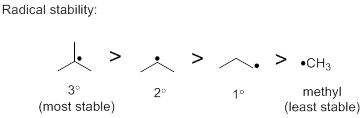
Selectivity determining step: The selectivity determining step is the breakage of the
b)
Interpretation:
The possible products containing two bromines that are not on the same carbon has to be predicted.
Concept Introduction:
Free radical stability: As the number of carbon substituents increases, the stability of the free radical increases.

Selectivity determining step: The selectivity determining step is the breakage of the
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Modified Mastering Chemistry With Pearson Etext -- Standalone Access Card -- For Fundamentals Of General, Organic, And Biological Chemistry (8th Edition)
- Given the balanced equation with an unknown compound represented by X, which compound is represented by X?arrow_forwardFor the following reactions, identify the atom(s) being oxidized and reduced:arrow_forwardThe following equation shows the reaction of baking soda (NaHCO3) and hydrochloric acid (HCl). NaHCO3+HCl → CO2+H2O+NaCl If you have 3.0 grams of NaHCO3, how many moles of HCl are needed for a complete relation?arrow_forward
- Identify the functional groups in the following compoundsarrow_forwardConsider the intermolecular forces present in a pure sample of each of the following compounds: CH₃CH₂OH and CH₃COCH₃. Identify the intermolecular forces that these compounds have in common.arrow_forwardThe standard heat of combustion of liquid methyl cyclopentane, C6H12(l),C6H12(l), was measured to be −3937.7 kJ/mol.−3937.7 kJ/mol. What is Δ?̂ ∘f C6H12(l),ΔH^f C6H12(l)∘, the standard heat of formation of liquid methyl cyclopentane?arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





