
(a)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 39E
Electron dot structure of
![]()
The structural formula of
![]()
Explanation of Solution
In molecule
![]()
Figure 1
![]()
Figure 2
Solid line, in Figure 2, between the iodine and oxygen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the atoms present in that bond.
An electron dot structure and structural formula of
(b)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 39E
Electron dot structure of
![]()
The structural formula of
![]()
Explanation of Solution
In molecule
![]()
Figure 3
![]()
Figure 4
Each solid line, in Figure 4, between the iodine and oxygen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the central atom iodine and the surrounding oxygen atom.
An electron dot structure and structural formula of
(c)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 39E
Electron dot structure of
![]()
The structural formula of
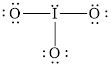
Explanation of Solution
In molecule
![]()
Figure 5

Figure 6
Each solid line, in Figure 6, between the iodine and oxygen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the central atom iodine and the surrounding oxygen atom.
An electron dot structure and structural formula of
(d)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 39E
Electron dot structure of
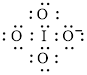
The structural formula of
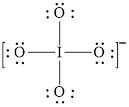
Explanation of Solution
In molecule

Figure 7

Figure 8
Each solid line, in Figure 8, between the iodine and oxygen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the central atom iodine and the surrounding oxygen atoms.
An electron dot structure and structural formula of
Want to see more full solutions like this?
Chapter 12 Solutions
INTRODUCTORY CHEMISTRY-STD.GDE.+SOL.MAN
- Given the electronegativity for O is 3.5 and S is 2.5 in Sulfate, would you predict the molecule to be polar or nonpolar"arrow_forwardConsider an iodine tetrafluoride cation (IF4+): What is the elemental symbol of its central atom?arrow_forward3-119 Perchloroethylene, which is a liquid at room temperature, is one of the most widely used solvents for commercial dry cleaning. It is sold for this purpose under several trade names, including Perciene®. Does this molecule have polar bonds? Is it a polar molecule? Does it have a dipole?arrow_forward
- 3-58 In Section 2-3B, we saw that there are seven diatomic elements. (a) Draw Lewis structures for each of these diatomic elements. (b) Which diatomic elements are gases at room temperature? Which are liquids? Which are solids?arrow_forward3-89 Is it possible for a molecule to have no polar bonds and yet have a dipole? Explain.arrow_forward(c) Theoretically, define the following elements in the formulae belowPth = ρghq x ŋarrow_forward
- What is the relationship between the tendency of a main-group element to form a monatomic ion and its position in the periodic table? In what part of the table are the main-group ele-ments that typically form cations? Anions?arrow_forwardWhich of the following would form an ionic compound with oxygen? A. Hydrogen B. Aluminum C. Manganese D. Selenium E. Iodinearrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning



