
Concept explainers
(a)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 44E
Electron dot structure and structural formula of

Explanation of Solution
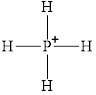
In molecule

Figure 1

Figure 2
Each solid line, in Figure 2, between the phosphorous atom and the hydrogen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the atoms present in that bond.
An electron dot structure and structural formula of
(b)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 44E
Electron dot structure and structural formula of
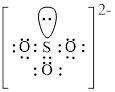
Explanation of Solution
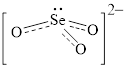
In molecule
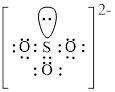
Figure 3

Figure 4
Each solid line, in Figure 4, between the oxygen atom and the selenium atom shows the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the atoms present in that bond.
An electron dot structure and structural formula of
(c)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 44E
Electron dot structure and structural formula of
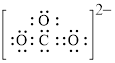
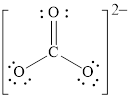
Explanation of Solution
In molecule
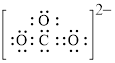
Figure 5

Figure 6
Each solid line, in Figure 6, between the carbon and oxygen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the atoms present in that bond.
An electron dot structure and structural formula of
(d)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 44E
Electron dot structure and structural formula of
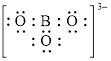
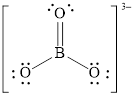
Explanation of Solution
In molecule
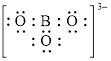
Figure 7

Figure 8
Each solid line, in Figure 8, between the boron and oxygen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the atoms present in that bond.
An electron dot structure and structural formula of
Want to see more full solutions like this?
Chapter 12 Solutions
INTRODUCTORY CHEMISTRY-STD.GDE.+SOL.MAN
- 3-58 In Section 2-3B, we saw that there are seven diatomic elements. (a) Draw Lewis structures for each of these diatomic elements. (b) Which diatomic elements are gases at room temperature? Which are liquids? Which are solids?arrow_forward2-98 Explain how the ionization energy of atoms changes when proceeding down a group of the Periodic Table and explain why this change occurs.arrow_forwardElements in the same group of the periodic table often formoxyanions with the same general formula. The anions arealso named in a similar fashion. Based on these observations,suggest a chemical formula or name, as appropriate, for eachof the following ions: (a) BrO4-, (b) SeO32-, (c) arsenate ion,(d) hydrogen tellurate ion.arrow_forward
- write the lewis structure of the molecule including the resonance forms, and the formal charges. the relative positions of the atoms are given as references answer #3arrow_forwardWhich one of the following does not occur as diatomic molecules in elemental form? Group of answer choices oxygen hydrogen sulfur nitrogen brominearrow_forwardAmmonium nitrate is an inexpensive source of nitrogen content used to enrichfertilizers. Although when handled improperly, it could be dangerous andcatastrophic. What is the chemical formula for ammonium nitrate?arrow_forward
- What is the chemical formula of the following compound? What would be possible identities of the anion and cation?arrow_forwardWrite the chemical formula of the following. A. Calcium chloride B. Calcium chloratearrow_forwardIf X is in the period 5, the ion formed has the same electron configuration as the noble gasarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning


