
(a)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 40E
Electron dot structure of
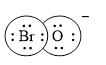
The structural formula of
![]()
Explanation of Solution
In molecule

Figure 1
![]()
Figure 2
Solid line, in Figure 2, between the bromine and oxygen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the atoms present in that bond.
An electron dot structure and structural formula of
(b)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 40E
Electron dot structure of
![]()
The structural formula of
![]()
Explanation of Solution
In molecule
![]()
Figure 3
![]()
Figure 4
Each solid line, in Figure 4, between the bromine and oxygen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the central atom bromine and the surrounding oxygen atom.
An electron dot structure and structural formula of
(c)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 40E
Electron dot structure of
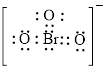
The structural formula of
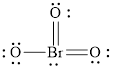
Explanation of Solution
In molecule
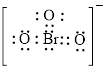
Figure 5

Figure 6
Each solid line, in Figure 6, between the bromine and oxygen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the central atom bromine and the surrounding oxygen atom.
An electron dot structure and structural formula of
(d)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 40E
Electron dot structure of
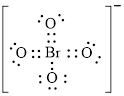
The structural formula of
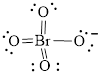
Explanation of Solution
In molecule
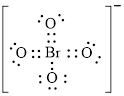
Figure 7
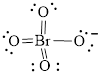
Figure 8
Each solid line in Figure 8, between the bromine and oxygen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the central atom bromine and the surrounding oxygen atoms.
An electron dot structure and structural formula of
Want to see more full solutions like this?
Chapter 12 Solutions
INTRODUCTORY CHEMISTRY-STD.GDE.+SOL.MAN
- Elements in the same group of the periodic table often formoxyanions with the same general formula. The anions arealso named in a similar fashion. Based on these observations,suggest a chemical formula or name, as appropriate, for eachof the following ions: (a) BrO4-, (b) SeO32-, (c) arsenate ion,(d) hydrogen tellurate ion.arrow_forwardWhat is the relationship between the tendency of a main-group element to form a monatomic ion and its position in the periodic table? In what part of the table are the main-group ele-ments that typically form cations? Anions?arrow_forward3C. and 3Harrow_forward
- In many ways, arsenate (AsO4 3−) is very similar to phosphate (PO4 3-), yet it does not substitute for phosphate in biomolecules. After reviewing the essential atomic characteristics of the element arsenic, explain this phenomenon.arrow_forwardGiven the following elements: Si, Sr, Cu, Ti, S (a) which of those elements would have the larget atomic radius? (b) which of those elements would have the highest ionization energy? (c) which of those elements would have the lowest electronegativity?arrow_forwardWhich one of the following does not occur as diatomic molecules in elemental form? Group of answer choices oxygen hydrogen sulfur nitrogen brominearrow_forward
- Give a cation isoelectronic with fluoride ion______________________ 3.Arrange in increasingsize: sodiumion, nitride ion, magnesium ion, oxide ion, neon. (explain your rationale)______<______<______<_____<_____ Arrange in decreasingsize: sulfide ion, argon, potassium ion, calcium ion, chloride ion. (explain your rationale)______>______>______>_____>___arrow_forwardIs polonium ionic or covalent ?arrow_forwardGive the electron dot diagram for N, O, S, H2O, and methane.arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning



