
Concept explainers
(a)
Interpretation:
The molecular structure of
Concept introduction:
The molecular structure can be determined by drawing Lewis structure and then counting total bond pairs and lone pairs and assigning the structure according to VSEPR theory.
(a)
Answer to Problem 5RQ
Molecular structure-Bent
Geometry-Tetrahedral
Explanation of Solution
Lewis structure of
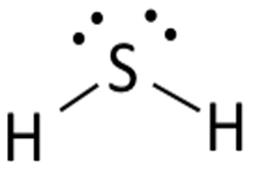
Structure shows that in
(b)
Interpretation:
The molecular structure of
Concept introduction:
The molecular structure can be determined by drawing Lewis structure and then counting total bond pairs and lone pairs and assigning the structure according to VSEPR theory.
(b)
Answer to Problem 5RQ
Molecular structure-Pyramidal
Geometry-Tetrahedral
Explanation of Solution
Lewis structure of
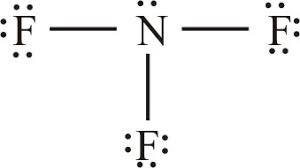
Structure shows that in
(c)
Interpretation:
The molecular structure of
Concept introduction:
The molecular structure can be determined by drawing Lewis structure and then counting total bond pairs and lone pairs and assigning the structure according to VSEPR theory
(c)
Answer to Problem 5RQ
Molecular structure- Trigonal planar
Geometry-Trigonal planar
Explanation of Solution
Lewis structure of
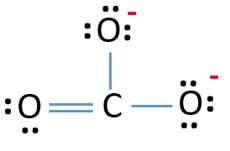
Structure shows that in
(d)
Interpretation:
The molecular structure of
Concept introduction:
The molecular structure can be determined by drawing Lewis structure and then counting total bond pairs and lone pairs and assigning the structure according to VSEPR theory
(d)
Answer to Problem 5RQ
Molecular structure- Tetrahedral
Geometry-Tetrahedral
Explanation of Solution
Lewis structure of
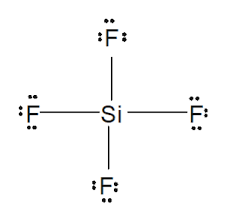
Structure shows that in
Chapter 12 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





