
Interpretation:
The strongest bond has to be identified from C=O bond in an ester (1735 cm-1) or the C=O bond in a saturated
Concept introduction:
Spectroscopy:
It is study of the interaction of matter and
IR frequency (cm-1):
It is the number of wave crests that pass by a given point in one second frequency has units of hertz (Hz).
Stretch vibrations:
It is a vibration occurring along the line of the bond a stretching vibration changes the bond length.
Bending vibrations:
It is a vibration that does not occur along the line of the bond, bending vibration changes the bond angle.
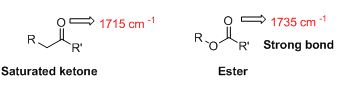
Given information:
The IR value is given below,
Trending nowThis is a popular solution!

Chapter 12 Solutions
Organic Chemistry
- The mass spectrum of 1-ethyl-1-methylcyclohexane shows many fragments, with two in very large abundance. Kne appears af m/z=111 and the other appears at m/z=97. Identify the structure of each of these fragments.arrow_forwardDetermine the Ksp of the following reactions. a. NaC2H3O2 ⇄ Na+ + C2H3O2- b. HBr⇄H++Br c. Zn(OH)2 ⇄ Zn+2 + 2HO-arrow_forward8 Give logical fragmentation reactions to account for the following ions observed in these mass spectra.(a) n-octane: 114, 85, 71, 57 (b) methylcyclohexane: 98, 83 (c) 2-methylpent-2-ene: 84, 69(d) pentan-1-ol: 70, 55, 41, 31arrow_forward
- Please help. Questions in images. Solvent Rf of A Rf of B Rf of C 10% ethyl acetate-90% hexanes .12 .07 .01 20% ethyl acetate-80% hexanes .34 .29 .10 30% ethyl acetate-70% hexanes .53 .42 .22 40% ethyl acetate-60% hexanes .72 .61 .35arrow_forward4 what is the ftir interpretation of 1-chloro-1-methylcyclohexane?arrow_forwardI'm not sure how to approach this problem. Do I look at the EWG's and EDG's of the substiuents in the products, or find the pka value of each product? please explain so I can do it myselfarrow_forward
- structures are fragments of major product of dehydration reaction of 2-methylbutan-2-ol. need to know why some of them have base peaks of 97. B and D have base peaks of 97.1, and A and C have peaks of 57.1arrow_forwardThe mass spectrum of 1-ethyl-1-methylcyclohexane shows many fragments, with two in very large abundance. One appears at m/z = 111 and the other appears at m/z = 97. Determine the identity and structure of each of these fragmentsarrow_forwardThe diasteromers of 4-t-butylcyclohexanol that you make can be distinguished by the splitting patterns of the proton attached to the carbon bearing th OH group. This is due to the fact that the H that is axial is a triplet of triplets because it is coupled to to sets of non-eqivalent protons, while the equatorial H is coupled to 4 equivalent hydrogens and is a pentet. True False??arrow_forward
- Describe stereoselective, regioselective, or chemoselective, the reactions below.arrow_forward2-Methyl-1-pentene has the prominent peaks at m/z 29, 41, 55, 69, and 84. Propose their structural formulas.arrow_forward9- Draw the 1H NMR spectrum of Ethyl bromide or 2-Chloropropane. Show the splittingindicating which is singlet, which is doublet, etc.arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

