
(a)
Interpretation:
The equilibrium pressure of
Concept introduction:
Equilibrium constant
In gas phase reactions, partial pressure is used to write equilibrium equation than molar concentration. Equilibrium constant
Consider the reaction where A reacts to give B.
On rearranging,
Where,
The pressure of each species of ideal gas and its molar concentration are directly proportional to each other.
In same way
Where,
R is gas constant
T is absolute temperature
(b)
Interpretation:
The given reaction is endothermic or exothermic has to be identified.
Concept introduction:
Le Chatelier's principle states that if a system in equilibrium gets disturbed due to modification of concentration, temperature, volume, and pressure, then it reset to counteract the effect of disturbance.
Effect of change in temperature:
Endothermic reaction: In this reaction increase in temperature which is absorbed in reactant side, the reaction occurs in a way to relieve the stress in reactant side. The reaction occurs along the direction of product side and increases equilibrium constant.
Exothermic reaction: In this reaction increase in temperature which is released in product side, the reaction occurs in a way to relieve the stress in product side. The reaction occurs along the direction of reactant side and decreases equilibrium constant.
(c)
Interpretation:
The calculated pressure of
Concept introduction:
Reaction quotient (Q):
Reaction quotient (Q) is the ratio of product of concentration of products to that of product of concentration of reactants, raised to the power of coefficients.
Consider a reaction
Where,
a, b, c and d are coefficients of each species.
t is arbitrary time for measuring concentration .
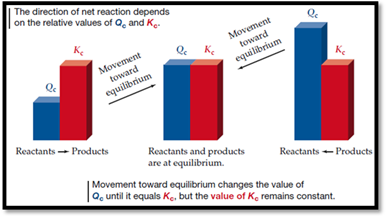
Figure 1
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
General Chemistry: Atoms First
- Describe a nonchemical system that is in equilibrium, and explain how the principles of equilibrium apply to the system.arrow_forwardShow that the complete chemical equation, the total ionic equation, and the net ionic equation for the reaction represented by the equation KI(aq)+I2(aq)KI3(aq) give the same expression for the reaction quotient. KI3 is composed of the ions K+ and I3-.arrow_forwardBecause calcium carbonate is a sink for CO32- in a lake, the student in Exercise 12.39 decides to go a step further and examine the equilibrium between carbonate ion and CaCOj. The reaction is Ca2+(aq) + COj2_(aq) ** CaCO,(s) The equilibrium constant for this reaction is 2.1 X 10*. If the initial calcium ion concentration is 0.02 AI and the carbonate concentration is 0.03 AI, what are the equilibrium concentrations of the ions? A student is simulating the carbonic acid—hydrogen carbonate equilibrium in a lake: H2COj(aq) H+(aq) + HCO}‘(aq) K = 4.4 X 10"7 She starts with 0.1000 AI carbonic acid. What are the concentrations of all species at equilibrium?arrow_forward
- The following equilibrium was studied by analyzing the equilibrium mixture for the amount of H2S produced. Sb2S3(s)+3H2(g)2Sb(s)+3H2S(g) A vessel whose volume was 2.50 L was filled with 0.0100 mol of antimony(III) sulfide, Sb2S3, and 0.0100 mol H2. After the mixture came to equilibrium in the closed vessel at 440C, the gaseous mixture was removed, and the hydrogen sulfide was dissolved in water. Sufficient lead(II) ion was added to react completely with the H2S to precipitate lead(II) sulfide, PbS. If 1.029 g PbS was obtained, what is the value of Kc at 440C?arrow_forwardThe diagram represents an equilibrium mixture for the reaction N2(g) + O2(g) ⇌ 2 NO(g) Estimate the equilibrium constant.arrow_forwardAt a certain temperature, K=0.29 for the decomposition of two moles of iodine trichloride, ICl3(s), to chlorine and iodine gases. The partial pressure of chlorine gas at equilibrium is three times that of iodine gas. What are the partial pressures of iodine and chlorine at equilibrium?arrow_forward
- Explain why there may be an infinite number of values for the reaction quotient of a reaction at a given temperature but theme can he only one value for the equilibrium constant at that temperature.arrow_forwardWrite the equilibrium constant expression for each reaction in terms of activities, simplifying where appropriate. a C(s)+O2(g)CO2(g) b P4(s)+5O2(g)P4O10(s) c 2HNO2(g)+3Cl2(g)2NCl3(g)+H2(g)+2O2(g)arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





