
(a)
Interpretation:
It is to be shown how a retrosynthetic analysis might be constructed for the given synthesis.
Concept introduction:
Retrosynthesis is the planning of
Answer to Problem 13.30P
The retrosynthesis for the given synthesis is
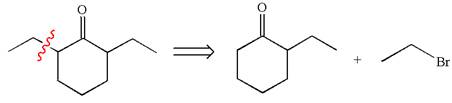
Explanation of Solution
The given synthetic reaction is
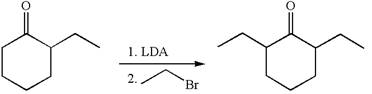
In the given synthesis, the product differs from the starting compound by one ethyl group bonded to six-membered ring. Thus, the bond between the ethyl group and ring carbon in the target must break to transform it to the starting compound.
Therefore, retrosynthesis for the given synthetic reaction is
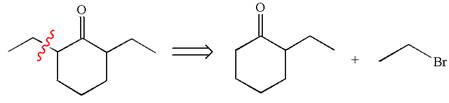
The retrosynthesis for the given synthetic reaction is shown by disconnecting the ethyl group from the ring.
(b)
Interpretation:
It is to be shown how a retrosynthetic analysis might be constructed for the given synthesis.
Concept introduction:
Retrosynthesis is the planning of organic synthesis, working backwards from target molecule to a simpler precursor, regardless of any interaction with reagents. Thus, the basis of retrosynthetic analysis is the transform, which means the reverse of a synthetic reaction. The precursors are the compounds, which are either readily available or easy to produce. The transform is indicated by an open arrow
Answer to Problem 13.30P
The retrosynthesis for the given synthesis is
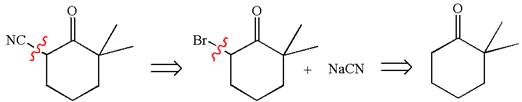
Explanation of Solution
The given synthetic reaction is
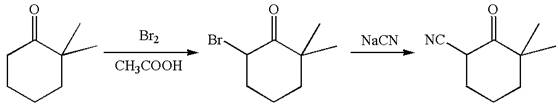
The given synthesis is of two steps; the final product is the target molecule. Thus, retrosynthesis could be planned from the target to the intermediate product to the starting material. The target molecule and the intermediate product differ in bromine and cyanide. Thus, the bond between the cyanide and ring carbon must break to transform into an intermediate. The intermediate and the starting molecule differ by bromine atom. Thus, the bond between the bromine and ring carbon must break to transform into the starting material.
Therefore, the retrosynthesis for the given synthetic reaction is
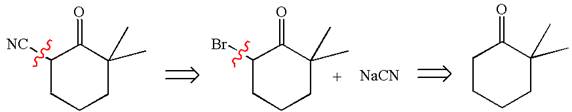
The retrosynthesis for the given synthetic reaction is shown by disconnecting the cyanide group and then by disconnecting the bromine atom from the ring.
(c)
Interpretation:
It is to be shown how a retrosynthetic analysis might be constructed for the given synthesis.
Concept introduction:
Retrosynthesis is the planning of organic synthesis, working backwards from target molecule to a simpler precursor, regardless of any interaction with reagents. Thus, the basis of retrosynthetic analysis is the transform, which means the reverse of a synthetic reaction. The precursors are the compounds, which are either readily available or easy to produce. The transform is indicated by an open arrow
Answer to Problem 13.30P
The retrosynthesis for the given synthesis is
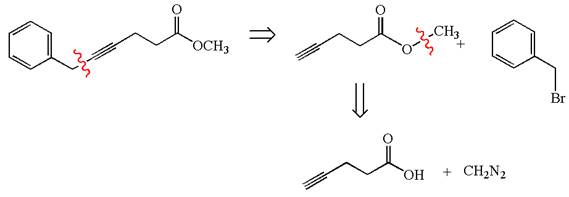
Explanation of Solution
The given synthetic reaction is
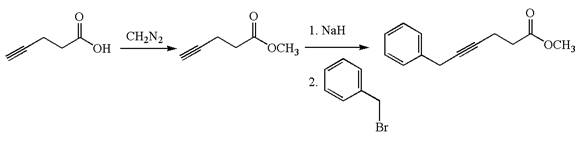
The given synthesis is of two steps; the final product is the target molecule. Thu, s the retrosynthesis could be planned from the target to the intermediate product to the starting material. The target molecule and the intermediate product differ in the benzyl group attached to the triple bonded carbon. Thus, the bond between the benzylic carbon and triple bonded carbon must break to transform into an intermediate. The intermediate can be transformed into the starting material by replacing the methyl group bonded to the oxygen atom by hydrogen.
Therefore, the retrosynthesis for the given synthetic reaction is
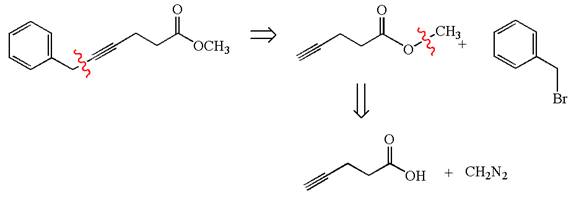
The retrosynthesis for the given synthetic reaction is shown by disconnecting the benzyl group from the triple bonded carbon and then by disconnecting the methyl group from the oxygen atom.
(d)
Interpretation:
It is to be shown how a retrosynthetic analysis might be constructed for the given synthesis.
Concept introduction:
Retrosynthesis is the planning of organic synthesis, working backwards from target molecule to a simpler precursor, regardless of any interaction with reagents. Thus, the basis of retrosynthetic analysis is the transform, which means the reverse of a synthetic reaction. The precursors are the compounds, which are either readily available or easy to produce. The transform is indicated by an open arrow
Answer to Problem 13.30P
The retrosynthesis for the given synthesis is
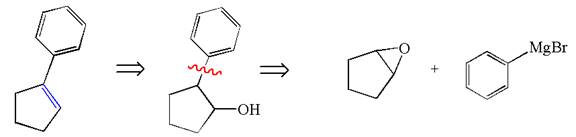
Explanation of Solution
The given synthetic reaction is
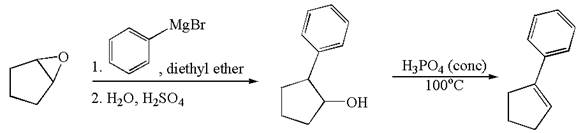
The given synthesis is of two steps; the final product is the target molecule. Thus the retrosynthesis could be planned from the target to the intermediate product to the starting material. The target molecule has a double bond, which is removed in the intermediate product having the ydroxyl group at that position. Thus, the
Therefore, the retrosynthesis for the given synthetic reaction is
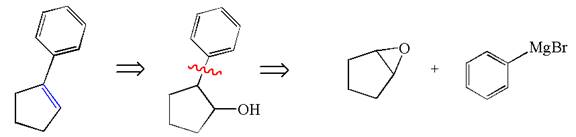
The retrosynthesis for the given synthetic reaction is shown by disconnecting the
(e)
Interpretation:
It is to be shown how a retrosynthetic analysis might be constructed for the given synthesis.
Concept introduction:
Retrosynthesis is the planning of organic synthesis, working backwards from target molecule to a simpler precursor, regardless of any interaction with reagents. Thus, the basis of retrosynthetic analysis is the transform, which means the reverse of a synthetic reaction. The precursors are the compounds, which are either readily available or easy to produce. The transform is indicated by an open arrow
Answer to Problem 13.30P
The retrosynthesis for the given synthesis is
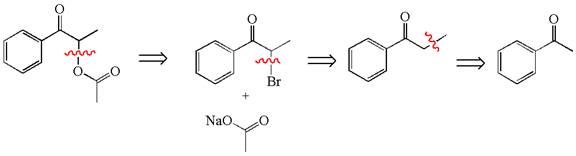
Explanation of Solution
The given synthetic reaction is
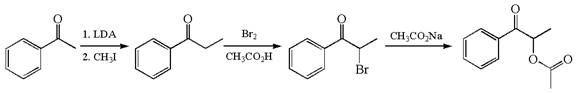
The given synthesis is of three steps; the final product is the target molecule. Thus, the retrosynthesis could be planned from the target to the second intermediate, then to the first intermediate, and finally into the starting material. The target molecule has the acetate group at the alpha position and the second intermediate has the bromine atom; thus, the bond between the alpha carbon and the oxygen of the acetate group must break. The second intermediate can be transformed into the first intermediate by breaking the bond between bromine and the alpha carbon. The first intermediate and the starting molecule differ by an additional methyl group; thus the bond between the alpha carbon and methyl must break to show the transform.
Therefore, the retrosynthesis for the given synthetic reaction is
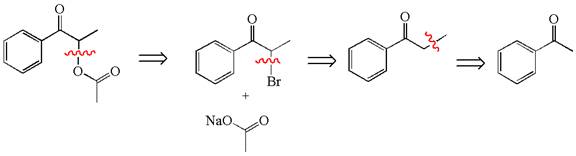
The retrosynthesis for the given synthetic reaction is shown by disconnecting appropriate bonds.
(g)
Interpretation:
It is to be shown how a retrosynthetic analysis might be constructed for the given synthesis.
Concept introduction:
Retrosynthesis is the planning of organic synthesis, working backwards from target molecule to a simpler precursor, regardless of any interaction with reagents. Thus, the basis of retrosynthetic analysis is the transform, which means the reverse of a synthetic reaction. The precursors are the compounds, which are either readily available or easy to produce. The transform is indicated by an open arrow
Answer to Problem 13.30P
The retrosynthesis for the given synthesis is
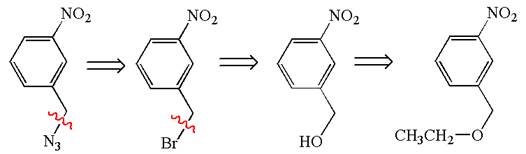
Explanation of Solution
The given synthetic reaction is
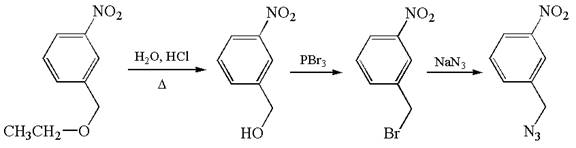
The given synthesis is of three steps; the final product is the target molecule. Thus; the retrosynthesis could be planned from the target to the second intermediate; then to the first intermediate; and finally into the starting material. The target can be transformed to the second intermediate by replacing the nitride group by bromine; thus the bond between the nitride group and carbon must break. The second intermediate can be transformed into the first intermediate by replacing the bromine by hydroxyl group; thus the bond between bromine and carbon must break. The first intermediate can be transformed into the starting material by replacing the hydroxyl group by ethoxy group.
Therefore, the retrosynthesis for the given synthetic reaction is
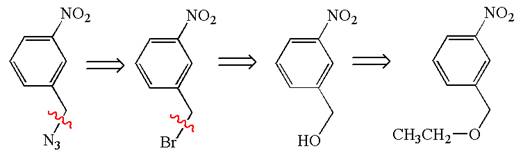
The retrosynthesis for the given synthetic reaction is shown by disconnecting appropriate bonds.
Want to see more full solutions like this?
Chapter 13 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Write a reasonable mechanism for the following transformation.arrow_forwardAcid anhydrides are generally formed by strongly heating an acid solution which promotes dehydration. Intramolecular acid dehydration create cyclic acid anhydrides .i. Besides providing energy for the reaction, how else does heating the acid solution promote the formation of the anhydride? )ii. Write a mechanism for the dehydration of the following molecule (Hint:remember that cyclohexyl rings can flip)arrow_forward#12. Outline a synthesis of the following molecules starting with ethyne and CH2I2 as the only carbon sources and any needed inorganic reagents. Obviously more than one ethyne molecule is needed for each of the syntheses.arrow_forward
- 1) How will you describe whether any compound has been oxidized or reduced? Support the answer with suitable examples. 2)Why carboxylic acid with a carbonyl group at 3rd position can be decarboxylated? 3) Explain why electrophilic aromatic substitution in Pyrrole takes place at C-2 positions whereas, in Pyridine it takes place at C-3 position? 4) List the following esters in order of decreasing reactivities towards hydrolysis with reason: Methyl benzoate, p-cyano methyl benzoate and p-hydroxy methyl benzoate 5)LDA is the base of choice for carbonyl compound to completely convert into enolate. Why?arrow_forwardA) Provide a reasonable multistep synthesis of the following molecule from the indicated starting material, using any reagents necessary. Please include both a retrosynthesis and a forward synthesis (that includes the product and reagents of each individual step in your synthesis). B) If you started with 1 mole of starting material and each step of your synthesis gave 100% yield, how many moles of the product drawn would you end with?arrow_forwardShow the electron-flow mechanism of the synthetic schemes (Aniline) of the picture below. This involves predicting major and by-products using electronic and structural effects. The arrow push mechanism must be shown.arrow_forward
- Below is a schematic representation of possible reactions that Compound X can undergo. Use the scheme to answer the following questions. A. What is the IUPAC name for Compound X? B. What type of reaction (s) is/are represented by (i) and (ii)? C. Compound X undergo transitions through either [A] or [B] to produce compounds [1], [2], [3] and [4]. Draw the structures of [A] and [B]. D. Illustrating with reaction mechanisms, show how compounds [1], [2], [3] and [4] are formed. E. Which of the compounds in the following pairs will occur in relatively higher yields and why? [1] and [2] [3] and [4] The attached image contains the scheme.arrow_forwardBelow is a schematic representation of possible reactions that Compound X can undergo. Use the scheme to answer the following questions. –a. What is the IUPAC name for Compound X? b. What type of reaction (s) is/are represented by (i) and (ii)? c. Compound X undergo transitions through either [A] or [B] to produce compounds [1], [2], [3] and [4]. Draw the structures of [A] and [B]. d. Illustrating with reaction mechanisms, show how compounds [1], [2], [3] and [4] are formed.e. Which of the compounds in the following pairs will occur in relatively higher yields and why?i. [1] and [2] ii. [3] and [4]arrow_forwardShow the electron-flow mechanism of the synthetic schemes (R-Salsolinol) of the picture below. This involves predicting major and by-products using electronic and structural effects. The arrow push mechanism must be shown.arrow_forward
- Determine a synthesis for the following molecule using organic reagents no larger than 2 or 3 carbons or benzene. You may use any inorganic reagents necessary. For each reaction cite a reference that shows the use of this type of reaction.arrow_forwardIn synthesis of benzoic acid with Grignard reagent, benzene is often detected as an impurity. How is benzene formed?arrow_forwardOutline a synthesis for the following transformation and provide a justification for your chosen strategy. (More than one steps may be required)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





