
Concept explainers
(a)
Interpretation:
It is to be determined whether the solvent would interfere with producing the intended target in the proposed synthetic step. For those that would, another solvent that can be used is to be suggested with an explanation.
Concept introduction:
Solvent plays an important role in the proposed synthetic step by not interfering with the reactant molecule or intermediates. For E2 elimination reactions, a strong base is used in the presence of a
Answer to Problem 13.33P
The solvent for the proposed synthetic step is appropriate. The proposed synthetic step is an example of an E2 reaction, which favors the Zaitsev product (most substituted alkene). If the solvent ethanol is deprotonated, the result is still an ethoxide ion which is the same base that is already shown.
Explanation of Solution
The given proposed synthetic step is:
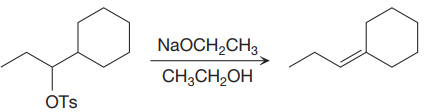
The reactant molecule has a good leaving group,
The requirement of the solvent for E2 reactions is aprotic, as aprotic solvents do not solvate the anions (negatively charged ions) as strongly as they solvate cations (positively charged ions).
(b)
Interpretation:
It is to be determined whether the solvent would interfere with producing the intended target in the proposed synthetic step. For those that would, another solvent that can be used is to be suggested with an explanation.
Concept introduction:
Solvent plays an important role in the proposed synthetic step by not interfering with the reactant molecule or intermediates. For E2 elimination reactions, a strong base is used in the presence of a polar aprotic solvent to yield the most substituted alkene is the major product. The requirement of the solvent for E2 reactions is aprotic, as aprotic solvents do not solvate the anions (negatively charged ions) as strongly as they solvate cations (positively charged ions). If the base used in the reaction and the base produced by the deprotonation of solvent molecules is the same, then the reaction proceeds in the forward direction and the proposed synthetic step is valid and acceptable. If the base used in the reaction is different from the base produced by the deprotonation of solvent molecules, then they would compete and the proposed synthetic route then would not be the valid route.
Answer to Problem 13.33P
The solvent ethanol for the proposed synthetic step is not appropriate. The proposed synthetic step is an example of an E2 reaction, which favors the Zaitsev product (most substituted alkene). The alkoxide ion shown
Explanation of Solution
The given proposed synthetic step is:
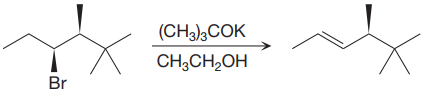
The reactant molecule has a good leaving group,
The requirement of the solvent for E2 reactions is aprotic, as aprotic solvents do not solvate the anions (negatively charged ions) as strongly as they solvate cations (positively charged ions).
(c)
Interpretation:
It is to be determined whether the solvent would interfere with producing the intended target in the proposed synthetic step. For those that would, another solvent that can be used is to be suggested with an explanation.
Concept introduction:
Solvent plays an important role in the proposed synthetic step by not interfering with the reactant molecule or intermediates.
For a substitution reaction which follows
Answer to Problem 13.33P
The solvent ethanol for the proposed synthetic step is not appropriate. The proposed synthetic step is an example of a
Explanation of Solution
The given proposed synthetic step is:
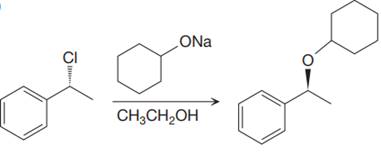
The reactant molecule has a moderate leaving group,
A
Thus, the solvent ethanol will interfere in the proposed synthetic step. Instead of ethanol cyclohexanol should be used, which will generate the same anion that is cyclohexanolate ion or any other aprotic solvent such as DMSO could be used.
The requirement of the solvent for
(d)
Interpretation:
It is to be determined whether the solvent would interfere with producing the intended target in the proposed synthetic step. For those that would, another solvent that can be used is to be suggested with an explanation.
Concept introduction:
Solvent plays an important role in the proposed synthetic step by not interfering with the reactant molecule or intermediates.
For a substitution reaction which follows
Answer to Problem 13.33P
The solvent for the proposed synthetic step is appropriate. The proposed synthetic step is an example of an E2 reaction, which favors the Zaitsev product (most substituted alkene). If the solvent ethanol is deprotonated, the result is still an ethoxide ion which is the same base that is already shown.
Explanation of Solution
The given proposed synthetic step is:
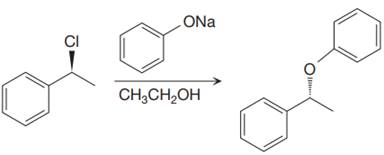
The reactant molecule has a good leaving group,
The requirement of the solvent for
Want to see more full solutions like this?
Chapter 13 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Basically, draw out what the product of this reaction would be. Thanks!arrow_forwardFor each of the following target molecules, design a multistep synthesis to show how it can be prepared from the given starting material:arrow_forward(time restraint!) Propose the best synthesis for each of the following molecules. All of the carbons in your target molecule MUST come from the attached sheet in this question.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
