
Concept explainers
(a)
Interpretation:
It is to be determined whether each of the following syntheses requires a reaction that alters the carbon skeleton.
Concept introduction:
Chemical syntheses are carries out by transforming one functional group to another. If the bonding arrangement of carbon atoms remains the same in the product formed, that means the synthesis does not require a change in the carbon skeleton. If it is changed, that means the synthesis requires a change in the carbon skeleton. The forming or breaking of carbon-carbon
Answer to Problem 13.29P
This chemical synthesis does require a reaction that alters the carbon skeleton because a carbon-carbon
Explanation of Solution
The given chemical synthesis is
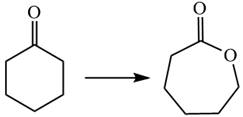
The starting compound is a six carbons ring whereas the product is a seven membered ring with one oxygen atom. Thus, the arrangement of carbon atoms in the product has changed by breaking the carbon-carbon
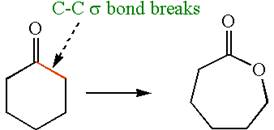
Therefore, this transformation requires a reaction that alters the carbon skeleton.
It is determined that the synthesis requires a reaction that alters the carbon skeleton based on the change in the arrangement of carbon atoms.
(b)
Interpretation:
It is to be determined whether each of the following syntheses requires a reaction that alters the carbon skeleton.
Concept introduction:
Chemical syntheses are carries out by transforming one functional group to another. If the bonding arrangement of carbon atoms remains the same in the product formed, that means the synthesis does not require a change in the carbon skeleton. If it is changed, that means the synthesis requires a change in the carbon skeleton. The forming or breaking of carbon-carbon
Answer to Problem 13.29P
This chemical synthesis does not require a reaction that alters the carbon skeleton as there is no need to break or form a carbon-carbon
Explanation of Solution
The given chemical synthesis is
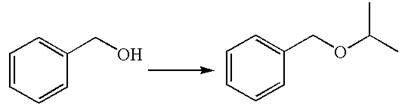
The starting compound is an alcohol, and the product is ether. The transformation occurs by replacement of the hydrogen attached to oxygen by the isopropyl group, which requires breaking of
It is determined that the synthesis does not require a reaction that alters the carbon skeleton based on the retention of the arrangement of carbon atoms.
(c)
Interpretation:
It is to be determined whether each of the following syntheses requires a reaction that alters the carbon skeleton.
Concept introduction:
Chemical syntheses are carries out by transforming one functional group to another. If the bonding arrangement of carbon atoms remains the same in the product formed, that means the synthesis does not require a change in the carbon skeleton. If it is changed, that means the synthesis requires a change in the carbon skeleton. The forming or breaking of carbon-carbon
Answer to Problem 13.29P
This chemical synthesis does require a reaction that alters the carbon skeleton because a carbon-carbon
Explanation of Solution
The given chemical synthesis is
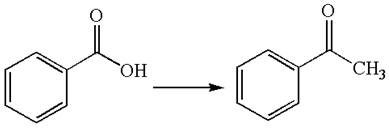
In this chemical synthesis, the carboxylic acid is converted to a
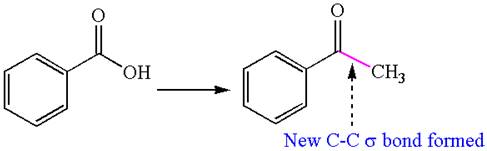
It means there is a formation of carbon-carbon
It is determined that the synthesis requires a reaction that alters the carbon skeleton based on the change in the arrangement of carbon atoms.
(d)
Interpretation:
It is to be determined whether each of the following syntheses requires a reaction that alters the carbon skeleton.
Concept introduction:
Chemical syntheses are carries out by transforming one functional group to another. If the bonding arrangement of carbon atoms remains the same in the product formed, that means the synthesis does not require a change in the carbon skeleton. If it is changed, that means the synthesis requires a change in the carbon skeleton. The forming or breaking of carbon-carbon
Answer to Problem 13.29P
This chemical synthesis does not require a reaction that alters the carbon skeleton because carbon-carbon
Explanation of Solution
The given chemical synthesis is
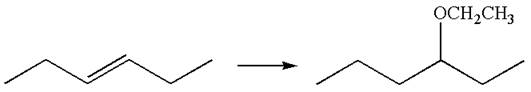
The starting compound is an
It is determined that the synthesis does not require a reaction that alters the carbon skeleton based on the retention of arrangement of carbon atoms.
(e)
Interpretation:
It is to be determined whether each of the following syntheses requires a reaction that alters the carbon skeleton.
Concept introduction:
Chemical syntheses are carries out by transforming one functional group to another. If the bonding arrangement of carbon atoms remains the same in the product formed, that means the synthesis does not require a change in the carbon skeleton. If it is changed, that means the synthesis requires a change in the carbon skeleton. The forming or breaking of carbon-carbon
Answer to Problem 13.29P
This chemical synthesis does require a reaction that alters the carbon skeleton because a carbon-carbon
Explanation of Solution
The given chemical synthesis is
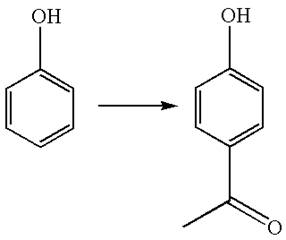
In this chemical synthesis, the hydrogen atom of benzene is replaced by the acetyl group,
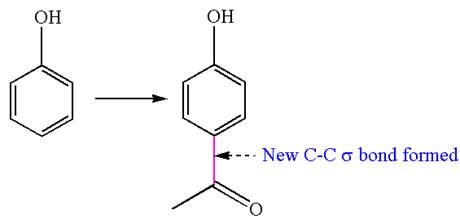
Therefore, this synthesis requires a reaction that alters the carbon skeleton.
It is determined that the synthesis requires a reaction that alters the carbon skeleton based on the change in the arrangement of carbon atoms.
(f)
Interpretation:
It is to be determined whether each of the following syntheses requires a reaction that alters the carbon skeleton.
Concept introduction:
Chemical syntheses are carries out by transforming one functional group to another. If the bonding arrangement of carbon atoms remains the same in the product formed, that means the synthesis does not require a change in the carbon skeleton. If it is changed, that means the synthesis requires a change in the carbon skeleton. The forming or breaking of carbon-carbon
Answer to Problem 13.29P
This chemical synthesis does require a reaction that alters the carbon skeleton because a carbon-carbon
Explanation of Solution
The given chemical synthesis is
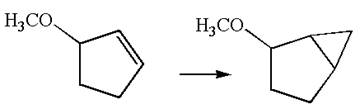
The starting compound has a five carbons ring with double bond, and the product has a five carbons ring fused with a three-membered ring. This could occur by breaking of carbon-carbon
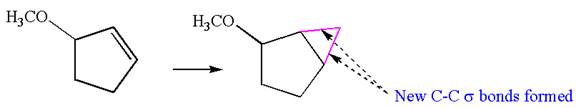
Therefore, this synthesis requires a reaction that alters the carbon skeleton.
It is determined that the synthesis requires a reaction that alters the carbon skeleton based on the change in arrangement of carbon atoms.
(g)
Interpretation:
This chemical synthesis does require a reaction that alters the carbon skeleton because a carbon-carbon
Concept introduction:
Chemical syntheses are carries out by transforming one functional group to another. If the bonding arrangement of carbon atoms remains the same in the product formed, that means the synthesis does not require a change in the carbon skeleton. If it is changed, that means the synthesis requires a change in the carbon skeleton. The forming or breaking of carbon-carbon
Answer to Problem 13.29P
No, this chemical synthesis does not require a reaction that alters the carbon skeleton because carbon-carbon
Explanation of Solution
The given chemical synthesis is
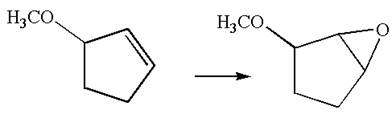
The starting compound is an alkene, and the product is
It is determined that the synthesis does not require a reaction that alters the carbon skeleton based on the retention of arrangement of carbon atoms.
(h)
Interpretation:
It is to be determined whether each of the following syntheses requires a reaction that alters the carbon skeleton.
Concept introduction:
Chemical syntheses are carries out by transforming one functional group to another. If the bonding arrangement of carbon atoms remains the same in the product formed, that means the synthesis does not require a change in the carbon skeleton. If it is changed, that means the synthesis requires a change in the carbon skeleton. The forming or breaking of carbon-carbon
Answer to Problem 13.29P
This chemical synthesis does require a reaction that alters the carbon skeleton because a carbon-carbon
Explanation of Solution
The given chemical synthesis is
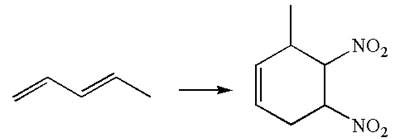
The starting compound has a five carbons chain with two conjugated double bonds, and the product has a six carbons ring fused. This could occur by breaking of carbon-carbon
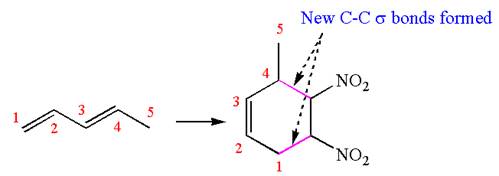
Therefore, this synthesis requires a reaction that alters the carbon skeleton.
It is determined whether the synthesis requires a reaction that alters the carbon skeleton based on the change in the arrangement of carbon atoms.
Want to see more full solutions like this?
Chapter 13 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Direction: predict the major new product if any resulting from the each of the following reactions please use this example as example. Dont use the stick form those are confusing.arrow_forwardShow how each of the following compounds can be synthesized from an alkene:arrow_forwardSynthesize the structure below. Please label which steps you have used to complete the synthesisarrow_forward
- The following four reactions will be the focus of the upcoming chapters (substitution and elimination reactions). Draw the curved arrows that accomplish each of the transformation shown:arrow_forwardShow other tautomeric form for each of following structure and state which form present equilibrium is far higher.arrow_forwardBased on the following reaction sequence, identify the major productarrow_forward
- Which of the following nucleophilic substitution reactions will take place?arrow_forwardFollow the curved arrows and draw the products of the following reaction. Include all lone pairs and charges as appropriate. Drawarrow_forwardCan you please help propose a syntheses for this molecule using the given reactant?arrow_forward
- Select reaction conditions that would allow you to carry out each of the following stereospecific transformations:arrow_forwardDraw the main organic product(s) formed in each of the following reactions:arrow_forwardComplete the following reactions by drawing the major product (sfor each of the following reactions. Unless specified, assume there is only on equivalent of the reactants.d) Please correct answer and don't use hend raitingarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
