
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13, Problem 41P
Interpretation Introduction
Interpretation:
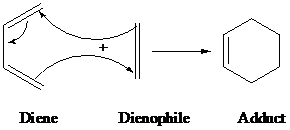
The structure for the adduct formed in the reaction between
Concept introduction:
Diels-alder is a type of organic reaction in which substituted
The driving force of the reaction is the formation of new σ-bonds, which are energetically more stable than the π-bonds.
The general reaction of Diels-alder is as follows:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Compound A was oxidized with periodic acid to give B, which after acid hydrolysis gave C. Bromine oxidation of C gave D. Suggest structural formulas, including stereochemistry, for compounds B, C, and D.
Compare reactivity of the following compounds: a) bromobenzene, b) benzyl bromide, c) ethyl bromide, d) vinyl bromide. Give explanations.
Birch reduction of 2-methoxynaphthalene gave a mixture of two isomeric compounds, each having the molecular formula C11H14O. Suggest reasonable structures for these compounds.
Chapter 13 Solutions
Organic Chemistry
Ch. 13 - Prob. 1PPCh. 13 - Prob. 2PPCh. 13 - Prob. 3PPCh. 13 - Practice Problem 13.4 From each set of resonance...Ch. 13 - Practice Problem 13.5 The following enol (an...Ch. 13 - Prob. 6PPCh. 13 - Practice Problem 13.7
Two compounds, A and B, have...Ch. 13 - Prob. 8PPCh. 13 - Prob. 9PPCh. 13 - Prob. 10PP
Ch. 13 - Prob. 11PPCh. 13 - Prob. 12PPCh. 13 - Prob. 13PPCh. 13 - Prob. 14PPCh. 13 - Prob. 15PPCh. 13 - Practice Problem 13.16
Diels–Alder reactions also...Ch. 13 - Prob. 17PPCh. 13 - Prob. 18PCh. 13 - What product would you expect from the following...Ch. 13 - Prob. 20PCh. 13 - Prob. 21PCh. 13 - Provide the reagents necessary for each of the...Ch. 13 - Prob. 23PCh. 13 - Prob. 24PCh. 13 - Prob. 25PCh. 13 - When 1-pentene reacts with N-bromosuccinimide...Ch. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - 13.31 Provide a mechanism that explains formation...Ch. 13 - 13.32 Provide a mechanism that explains formation...Ch. 13 - Treating either 1-chloro-3-methyl-2-butene or...Ch. 13 - Prob. 34PCh. 13 - Prob. 35PCh. 13 - Although both 1-bromobutane and 4-bromo-1-butene...Ch. 13 - Prob. 37PCh. 13 - Prob. 38PCh. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - 13.44 When furan and maleimide undergo a...Ch. 13 - Two controversial hard insecticides are aldrin and...Ch. 13 - Prob. 46PCh. 13 - Prob. 47PCh. 13 - Prob. 48PCh. 13 - Prob. 49PCh. 13 - Prob. 50PCh. 13 - Explain the product distribution below based on...Ch. 13 - Mixing furan (Problem 13.44) with maleic anhydride...Ch. 13 - Prob. 53PCh. 13 - Prob. 54PCh. 13 - Prob. 1LGPCh. 13 - Prob. 2LGP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Compound A, C3H7Br, does not react with cold dilute potassium permanganate solution. Upon treatment with potassium hydroxide in ethanol, A gives only product B, C3H6. Unlike A, B decolourises potassium permanganate solution. Ozonolysis of Bgives C, C2H4O, and D, CH2O. Suggest the structural formulae of A, B, C and D.Write the equations for all the reactions involved.arrow_forwardProvide the structure of the major organic product in the substitution reaction of 3-iodocyclopentene with water.arrow_forwardAnswer the following question pertaining to the reaction of maleic anhydride and 1,3-butadiene: - Which conformation of 1,3-butadiene, s-cis or s-trans, is thermodynamically preferred and why?arrow_forward
- Starting with acid chloride with exactly 5 carbon atoms, and using appropriate reagents outline the synthesis of the following molecules:arrow_forwardOutline the synthesis of 1-bromo-2-methylpropane by using suitable alkenearrow_forwardOne step in the synthesis of dodecahedrane involves reaction of the tetraene C with dimethylacetylene dicarboxylate (D) to afford two compounds having molecular formula C16H16O4. This reaction has been called a domino Diels–Alder reaction. Identify the two products formed.arrow_forward
- Give the reagents and conditions necessary for the following conversion. A to B, B to D, B to C, B to E, E to F, E to G, G to H, H to I. Hence deduce the name and structural formula of the compounds C and I. Compare the procedure for converting F and E to G.arrow_forwardFriedel–Crafts acylation of the individual isomers of xylene with acetyl chloride and aluminum chloride yields a single product, different for each xylene isomer, in high yield in each case. Write the structures of the products of acetylation of o-, m-, and p-xylene.arrow_forwardAnswer the following question pertaining to the reaction of maleic anhydride and 1,3-butadiene: - Specify the conformation required to enable a 1,3-diene to undergo the Diels-Alderreactionarrow_forward
- Diisopinocampheylborane (Ipc2BH) is a chiral organoborane, readily employed for the production of many asymmetric products used in total synthesis. It is a crystalline material that can be prepared as a single enantiomer via the hydroboration of two equivalents of α-pinene with borane.Explain why only one enantiomer of Ipc2BH is formed.arrow_forwardGive the structure of the following compound(a) isobutyl bromidearrow_forwardCompound A(C10H12O)gives off oxygen on treatment with sodium metal and also decolorizes Br2 in CCl4 to give organic compound B. Compound A on treatment with I2 in NaOH gives iodoform and salt C which after acidification gives a white solid D(C7H6O2). Using knowledge of organic chemistry identify structures A,B,C and Darrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
